77869
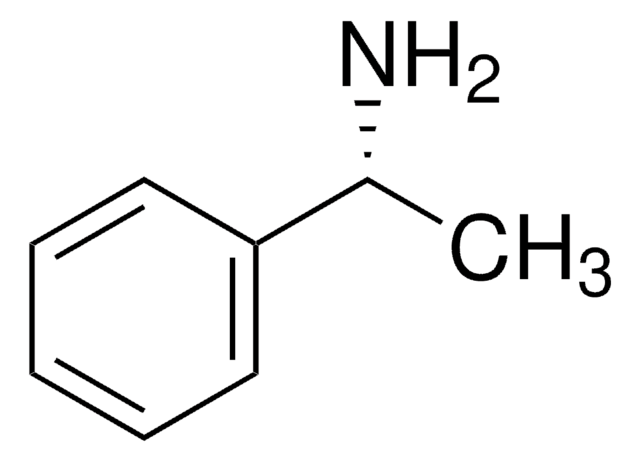
(S)-(−)-α-Methylbenzylamine
for chiral derivatization, LiChropur™, ≥99.0%
동의어(들):
(S)-(−)-1-Phenylethylamine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5CH(CH3)NH2
CAS Number:
Molecular Weight:
121.18
Beilstein:
2204907
EC Number:
MDL number:
UNSPSC 코드:
12000000
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
for chiral derivatization
Quality Level
vapor pressure
0.5 mmHg ( 20 °C)
분석
≥99.0% (sum of enantiomers, GC)
≥99.0%
형태
liquid
광학 활성
[α]20/D −30±1°, c = 10% in ethanol
광학 순도
enantiomeric ratio: ≥99.5:0.5 (GC)
품질
LiChropur™
기술
HPLC: suitable
refractive index
n20/D 1.526 (lit.)
n20/D 1.528
bp
187 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
C[C@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m0/s1
InChI key
RQEUFEKYXDPUSK-ZETCQYMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(S)-(−)-α-Methylbenzylamine is a chiral derivatizing agent, which is employed for derivatizing enantiomers into diastereoisomers.
애플리케이션
(S)-(−)-α-Methylbenzylamine may be used as a chiral derivatizating reagent for the determination of acetyl-D-carnitine (D-AC) in acetyl-L-carnitine (L-AC) using high-performance liquid chromatographic (HPLC) enantioseparation method.
법적 정보
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point (°F)
158.0 °F - closed cup
Flash Point (°C)
70 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Alejandra León et al.
Journal of natural products, 75(5), 859-864 (2012-05-12)
The enantiomeric lactams (-)-8, (+)-8, (+)-9, and (-)-9 were formed by the reaction of the dimeric phthalide rac-tokinolide B (rac-3) with (R)-(+)-α-methylbenzylamine and (S)-(-)-α-methylbenzylamine. The absolute configurations of compounds 8 and 9 were assigned by experimental and theoretically calculated electronic
Paul E Harrington et al.
Current medicinal chemistry, 14(28), 3027-3034 (2008-01-29)
The calcium sensing receptor (CaR) is a G protein-coupled receptor (GPCR) that plays a fundamental role in serum calcium homeostasis. The CaR is expressed on the chief cells of the parathyroid gland and is responsible for controlling the secretion of
Antoine Fadel et al.
The Journal of organic chemistry, 72(5), 1780-1784 (2007-01-30)
Enantiomerically pure (R)-(+)-pipecolic acid was synthesized in four steps and 42% overall yield starting from dihydropyran and (R)-alpha-methylbenzylamine. A general short strategy is also described for preparing (S)-proline (47.5% overall yield) and derivatives.
Carlos Fernandes et al.
The Journal of organic chemistry, 74(8), 3217-3220 (2009-03-17)
A short, convenient, gram scale protocol has been established to allow facile access to all four stereoisomers of 2-aminocyclobutanecarboxylic acid, each in enantiomerically pure form (ee >99%). Starting from the readily available cis racemate, the procedure combines efficient alpha-phenylethylamine derivative
Abraham R Martin et al.
Applied microbiology and biotechnology, 76(4), 843-851 (2007-06-22)
Enzyme immobilization often improves process economics, but changes in kinetic properties may also occur. The immobilization of a recombinant thermostable (S)-aminotransferase was made by entrapment on calcium alginate-3% (w/v)-and tested with (S)-(-)-(alpha)-methylbenzylamine for acetophenone production. The best immobilization results were
Chromatograms
HPLC Analysis of α-Methylbenzylamine Enantiomers (DANSYL Derivatives) on Astec® CYCLOBOND I 2000 DMP
application for HPLCapplication for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.