71478
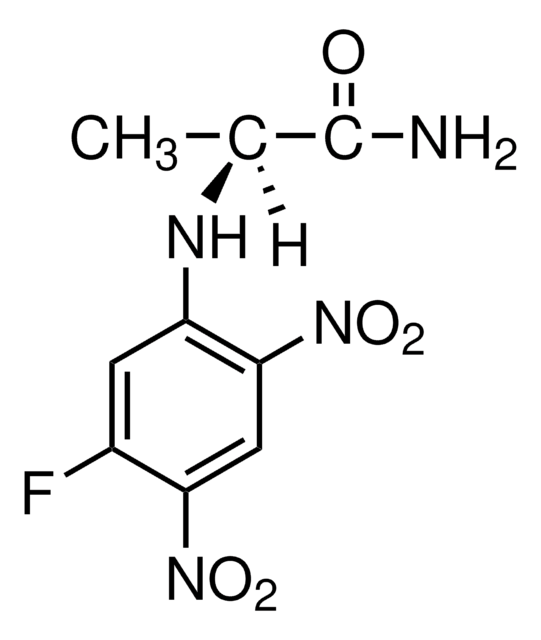
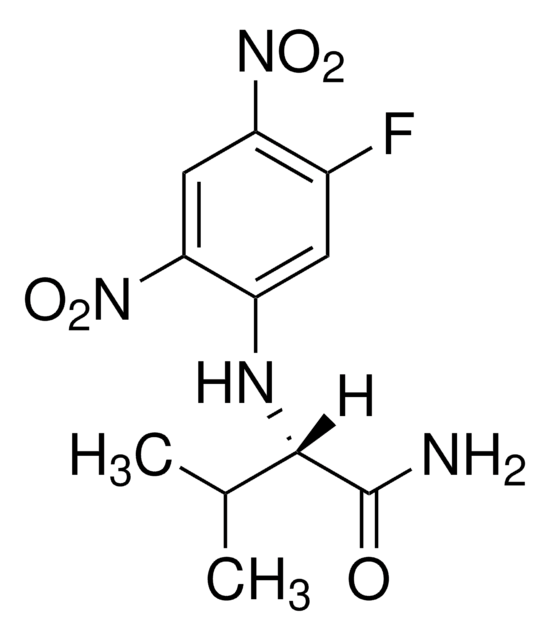
Nα-(2,4-Dinitro-5-fluorophenyl)-L-alaninamide
for chiral derivatization, LiChropur™, ≥99.0%
동의어(들):
FDAA, Marfey’s reagent
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C9H9FN4O5
CAS Number:
Molecular Weight:
272.19
Beilstein:
6820069
MDL number:
UNSPSC 코드:
12000000
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
derivatization grade ((chiral))
for chiral derivatization
Quality Level
분석
≥99.0% (sum of enantiomers, TLC)
≥99.0%
양식
powder
광학 활성
[α]20/D +56±2°, c = 1% in acetone
광학 순도
enantiomeric ratio: ≥99.5:0.5 (HPLC)
품질
LiChropur™
기술
HPLC: suitable
저장 온도
2-8°C
SMILES string
C[C@H](Nc1cc(F)c(cc1[N+]([O-])=O)[N+]([O-])=O)C(N)=O
InChI
1S/C9H9FN4O5/c1-4(9(11)15)12-6-2-5(10)7(13(16)17)3-8(6)14(18)19/h2-4,12H,1H3,(H2,11,15)/t4-/m0/s1
InChI key
NEPLBHLFDJOJGP-BYPYZUCNSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Nα-(2,4-Dinitro-5-fluorophenyl)-L-alaninamide (FDAA) is a chiral derivatizing agent (CDA), has high enantioselectivity but low sensitivity as compared to other CDAs. It is generally used to assign the stereochemistry of amino acids in trace amounts.
애플리케이션
FDAA was used as derivatizing reagent, in a study performed to understand unusual amino acids using reversed phase high performance liquid chromatography-electrospray ionization mass spectrometry (RPHPLC-ESI-MS).
기타 정보
Derivatization reagent for the assay of unusual chiral α-amino acid analogs
법적 정보
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatography-electrospray ionization mass spectrometry.
Hess S
Journal of Chromatography A, 1035(2), 211-219 (2004)
R Bhushan et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 879(29), 3148-3161 (2011-07-09)
The present paper describes an updated knowledge and status on Marfey's reagent (MR), 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (FDNP-L-Ala-NH(2)). The reagent is used for pre-column derivatization of amino acids followed by HPLC separation of the diastereomers so formed. Emphasis is put on the
J G Adamson et al.
Analytical biochemistry, 202(1), 210-214 (1992-04-01)
A chromatographic assay has been developed to quantitate racemization occurring during attachment of protected amino acids to peptide synthesis resins. Acidolytic cleavage of deprotected amino acids from supports and subsequent derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (Marfey's reagent) gave diastereomers separable by
S Kochhar et al.
Analytical biochemistry, 178(1), 17-21 (1989-04-01)
Amino acids are quantitatively determined by precolumn derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide and reversed-phase high-performance liquid chromatography with photometric detection at 340 nm. Excellent chromatographic resolution of a mixture of the derivatives of 20 amino acids including proline and cystine is
A D Tran et al.
Journal of chromatography, 516(1), 241-249 (1990-09-07)
Separation of amino acid enantiomers and peptide isomers has been made possible through the use of Marfey's reagent and high-performance capillary electrophoresis (HPCE). Samples of amino acids and peptides were first derivatized with Marfey's reagent and subsequently analyzed by HPCE.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.