63174
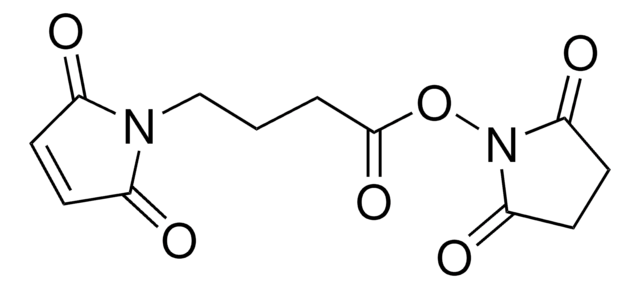
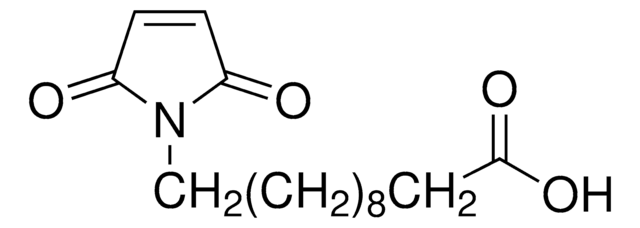
4-Maleimidobutyric acid
≥98.0% (T)
동의어(들):
γ-Maleimidobutyric acid, 4-Maleimidobutanoic acid, N-(3-Carboxypropyl)maleimide, N-Maleoyl-4-aminobutyric acid, N-Maleoyl-GABA
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C8H9NO4
CAS Number:
Molecular Weight:
183.16
Beilstein:
1455876
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
NACRES:
NA.25
추천 제품
애플리케이션
Gamma-maleimidobutyric acid may be used as a spacer in the construction of drug and other types of bioconjugates. 4-Maleimidobutyric acid is used with N-hydroxysuccinimide ester as a bifunctional cross-linking agent.
Modification reagent for thiol groups in proteins
기타 정보
SH-label for the modification of peptides and proteins; Used for the preparation of a new bleomycin analog for enzyme immunoassay (EIA); probe for membrane SH-groups
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
K Fujiwara et al.
Cancer research, 41(10), 4121-4126 (1981-10-01)
An antibody directed toward pepleomycin, a new antitumor antibiotic related structurally to bleomycin, has been produced in rabbits by immunization with a pepleomycin-protein conjugate which was prepared by a novel procedure of coupling pepleomycin to mercaptosuccinylated bovine serum albumin using
Biochemical Society Transactions, 11, 753-753 (1983)
N-polymethylenecarboxymaleimides -- a new class of probes for membrane sulphydryl groups.
D G Griffiths et al.
FEBS letters, 134(2), 261-263 (1981-11-16)
Marie Pribylova et al.
International journal of pharmaceutics, 415(1-2), 175-180 (2011-06-15)
A new targeted conjugates in which paclitaxel was used as a cytostatic compound and an analog of the gonadotropin-releasing hormone (GnRH) as a targeting moiety were synthesized. The molecule of the peptide hormone GnRH was modified to allow its connection
Weibo Cai et al.
Nature protocols, 3(1), 89-96 (2008-01-15)
To take full advantage of the unique optical properties of quantum dots (QDs) and expedite future near-infrared fluorescence (NIRF) imaging applications, QDs need to be effectively, specifically and reliably directed to a specific organ or disease site after systemic administration.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.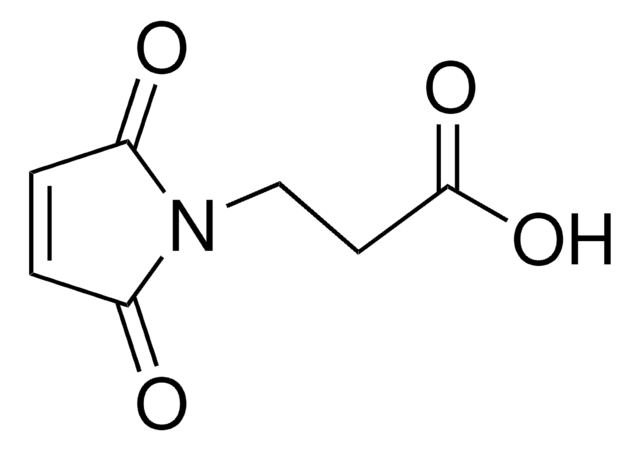

![O-[2-(Boc-amino)ethyl]-O′-(2-maleimidoethyl)ethylene glycol ≥96.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/270/188/cd3145b1-0ad3-409b-85c2-73937e007f04/640/cd3145b1-0ad3-409b-85c2-73937e007f04.png)