추천 제품
Grade
ACS reagent
Quality Level
vapor density
2.5 (vs air)
vapor pressure
2.7 mmHg ( 20 °C)
분석
≥99.8%
형태
liquid
autoignition temp.
833 °F
expl. lim.
15.2 %
기술
electrophoresis: suitable
불순물
≤0.0005 meq/g Titr. acid
≤0.003 meq/g Titr. base
≤0.15% water
증발 잔류물
≤0.005%
색상
APHA: ≤15
refractive index
n20/D 1.430 (lit.)
pH
6.7
bp
153 °C (lit.)
mp
−61 °C (lit.)
solubility
water: miscible
density
0.944 g/mL (lit.)
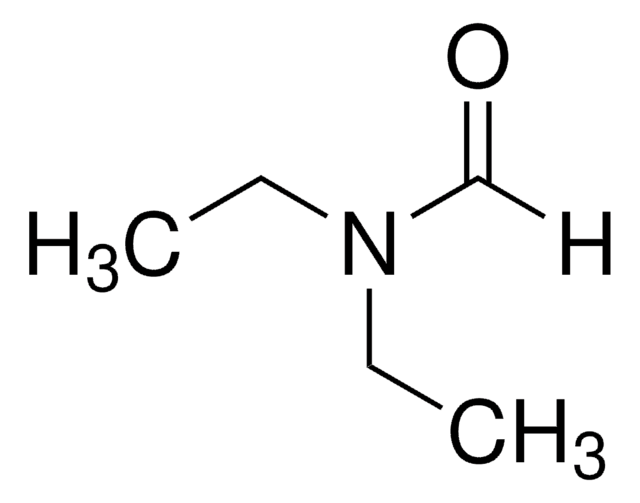
SMILES string
[H]C(=O)N(C)C
InChI
1S/C3H7NO/c1-4(2)3-5/h3H,1-2H3
InChI key
ZMXDDKWLCZADIW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N,N-Dimethylformamide (DMF) is a polar organic solvent with a wide range of industrial applications. It is a precursor for numerous reactions such as formylation, aminocarbonylation, amination, amidation and cyanation. Along with phosphorus oxychloride, it forms Vilsmeier reagent used in the formylation of reactive aromatic and heteroaromatic substrates. Dielectric relaxation experiments on DMF have been conducted between 238.15K and 338.15K. The thermophysical properties of the molecular interactions between DMF and the ionic liquids, ammonium salts and imidazolium salts have been studied. The characteristics of solute-solvent interactions between ammonium nitrate and DMF have been investigated.
애플리케이션
N,N-Dimethylformamide (DMF) may be used in the following processes:
- As a solvent synthesis of gold nanoparticles.
- As a solvent in capillary electrophoresis.
- Regioselective synthesis of N,N-dimethylaminopyridzines by reacting with choropyridazines.
- Synthesis of urea-N,N-dimethylformamide, a 3:1 solvate with urea.
Solvent for many hydrophobic organic compounds.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
135.5 °F - closed cup
Flash Point (°C)
57.5 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Preparation of gold nanoparticles in formamide and N,N-dimethylformamide in the presence of poly (amidoamine) dendrimers with surface methyl ester groups.
Esumi K, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 189(1), 155-161 (2001)
Reaction of chloropyridazines with N,N -dimethylformamide.
Lee WS, et al.
Journal of Heterocyclic Chemistry, 37(6), 1591-1591 (2000)
N,N-dimethylformamide as a reaction medium for metal nanoparticle synthesis.
Pastoriza-Santos I and Liz-Marzan LM.
Advances in Functional Materials, 19(5), 679-688 (2009)
Urea-N,N-dimethylformamide (3/1).
Fernandes P, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(12), 4861-4861 (2007)
Capillary electrophoresis in N,N-dimethylformamide.
Porras SP and Kenndler E.
Electrophoresis, 26(17), 3279-3291 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.