About This Item
추천 제품
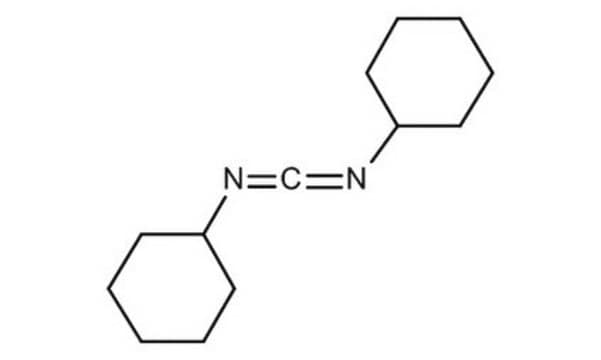
product name
DCC, puriss., ≥99.0% (GC)
grade
puriss.
Quality Level
분석
≥99.0% (GC)
형태
solid
반응 적합성
reaction type: Coupling Reactions
bp
122-124 °C/6 mmHg (lit.)
mp
32.0-37.0 °C
34-35 °C (lit.)
solubility
methylene chloride: 0.1 g/mL, clear, colorless
응용 분야
peptide synthesis
SMILES string
C1CCC(CC1)N=C=NC2CCCCC2
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
InChI key
QOSSAOTZNIDXMA-UHFFFAOYSA-N
유전자 정보
human ... EPHX2(2053)
mouse ... Ephx2(13850)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
It may be also used to synthesize:
- 1,3-Thiazetedine derivatives via [2+2] cycloaddition with 2-phenylethenyl- and 2-(4-nitrophenyl)ethenyl isothiocyanates.
- 1,3,5-Oxadiazine-4-thiones via [4+2] cycloaddition with benzoyl isothiocyanates.
- Sterically hindered 1,3,4-oxadiazole derivatives by reacting with (N-isocyanimino)triphenylphosphorane the presence of aromatic (or heteroaromatic) carboxylic acids.
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
이미 열람한 고객
문서
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.