33977
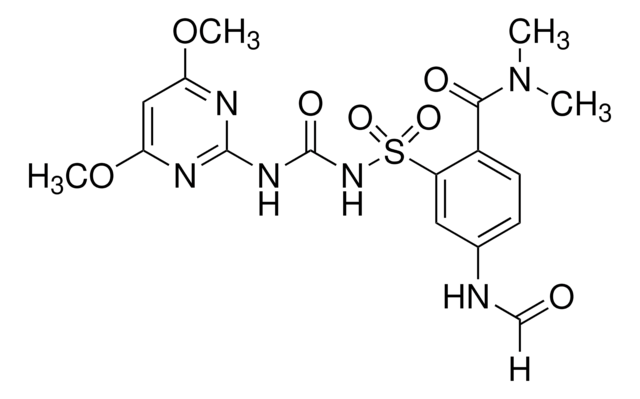
Foramsulfuron
PESTANAL®, analytical standard
동의어(들):
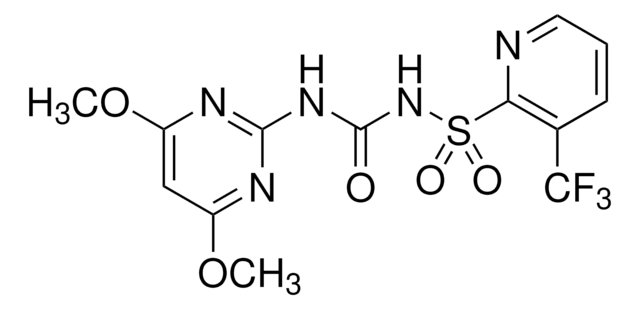
2-[3-(4,6-Dimethoxy-2-pyrimidinyl)ureidosulfonyl]-4-(formamido)-N,N-dimethylbenzamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C17H20N6O7S
CAS Number:
Molecular Weight:
452.44
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
제품 라인
PESTANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
agriculture
environmental
형식
neat
SMILES string
[H]C(=O)Nc1ccc(C(=O)N(C)C)c(c1)S(=O)(=O)NC(=O)Nc2nc(OC)cc(OC)n2
InChI
1S/C17H20N6O7S/c1-23(2)15(25)11-6-5-10(18-9-24)7-12(11)31(27,28)22-17(26)21-16-19-13(29-3)8-14(20-16)30-4/h5-9H,1-4H3,(H,18,24)(H2,19,20,21,22,26)
InChI key
PXDNXJSDGQBLKS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Foramsulfuron is a sulfonylurea herbicide used in controlling a wide spectrum of weeds. Its mode of action involves inhibiting the enzyme activity of acetolactate synthase (ALS) which is involved in biosynthesis of branched amino acids.
애플리케이션
Foramsulfuron may be used as a reference standard for the determination of foramsulfuron in water samples by solid-phase extraction and liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
법적 정보
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
James T Brosnan et al.
Planta, 243(1), 149-159 (2015-09-12)
This is a first report of an Ala-205-Phe substitution in acetolactate synthase conferring resistance to imidazolinone, sulfonylurea, triazolopyrimidines, sulfonylamino-carbonyl-triazolinones, and pyrimidinyl (thio) benzoate herbicides. Resistance to acetolactate synthase (ALS) and photosystem II inhibiting herbicides was confirmed in a population of
Photooxidation of foramsulfuron: Effects of char substances.
Vittoria PM, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 326, 16-20 (2016)
Trace analysis of sulfonylurea herbicides in water samples by solid-phase extraction and liquid chromatography-tandem mass spectrometry.
Fenoll J
Talanta, 101, 273-282 (2012)
Amit Paporisch et al.
Pesticide biochemistry and physiology, 138, 22-28 (2017-05-01)
Three sweet corn genotypes, two inbred lines (IBER001 and IBER002) and their hybrid (ER00X), differ in their phenotypic responses to several P450-metabolized herbicides, used in sweet corn, namely, foramsulfuron, iodosulfuron, rimsulfuron and tembotrione. Foramsulfuron is a sulfonylurea herbicide commonly formulated
Maykel Hernández-Mesa et al.
Journal of chromatography. A, 1617, 460831-460831 (2020-01-18)
This work proposes a novel Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method in combination with ultra-high performance liquid chromatography-tandem mass spectrometry for the determination of sulfonylurea residues in edible seeds. The chromatographic separation of nine sulfonylureas was accomplished
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.