추천 제품
Grade
analytical standard
Quality Level
제품 라인
PESTANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
agriculture
environmental
형식
neat
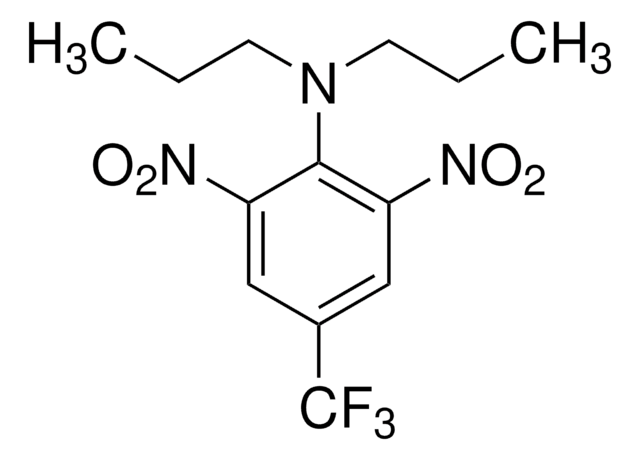
SMILES string
CCCN(CCC)c1c(cc(cc1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O
InChI
1S/C13H16F3N3O4/c1-3-5-17(6-4-2)12-10(18(20)21)7-9(13(14,15)16)8-11(12)19(22)23/h7-8H,3-6H2,1-2H3
InChI key
ZSDSQXJSNMTJDA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Trifluralin is a dinitroaniline herbicide used mostly as antiparasitic agent. It is used in controlling weeds and is characterized as microtubule-depolymerizing chemical.
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Trifluralin may have been used as standard in mass spectrometry employing negative chemical
ionization (GC-MS-NCI) technique for qualitative and quantitative analysis of trifluralin from soil extract samples.
ionization (GC-MS-NCI) technique for qualitative and quantitative analysis of trifluralin from soil extract samples.
법적 정보
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
212.0 °F - closed cup
Flash Point (°C)
100.00 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide.
Fernandes, Thais CC, Dania Elisa C. Mazzeo, and Maria A. Marin-Morales.
Pesticide Biochemistry and Physiology, 88.3, 252-259 (2007)
Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin.
Chan, Marion Man-Ying, and Dunne Fong.
Science, 249.4971, 924-924 (1990)
Quantitative confirmation of ethalfluralin and trifluralin in soil extracts by negative chemical ionization mass spectrometry.
Moyer, James R., and James L. Elder.
Journal of Agricultural and Food Chemistry, 32.4, 866-868 (1984)
R A Cardoso et al.
Genetics and molecular research : GMR, 9(1), 231-238 (2010-03-04)
Some herbicides are suspected of promoting teratogenic, carcinogenic and mutagenic events. Detection of induced mitotic crossing-over has proven to be an indirect way of testing the carcinogenic properties of suspicious substances, because mitotic crossing-over is involved in the multistep process
Perihan Binnur Kurt-Karakus et al.
Environmental toxicology and chemistry, 30(7), 1539-1548 (2011-04-08)
Concentrations of current-use pesticides (CUPs) in water, zooplankton, precipitation, and air samples as well as stereoisomer fractions (SF; herbicidally active/total stereoisomers) of metolachlor were determined in water samples collected from 10 remote inland lakes in Ontario, Canada, between 2003 and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.