00370580
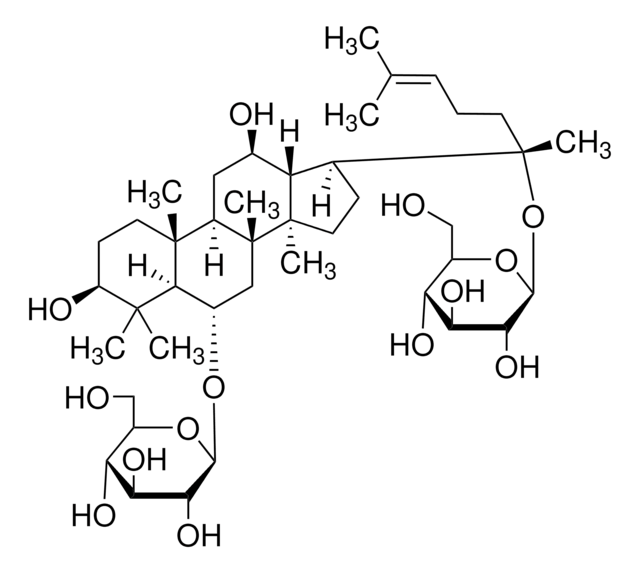
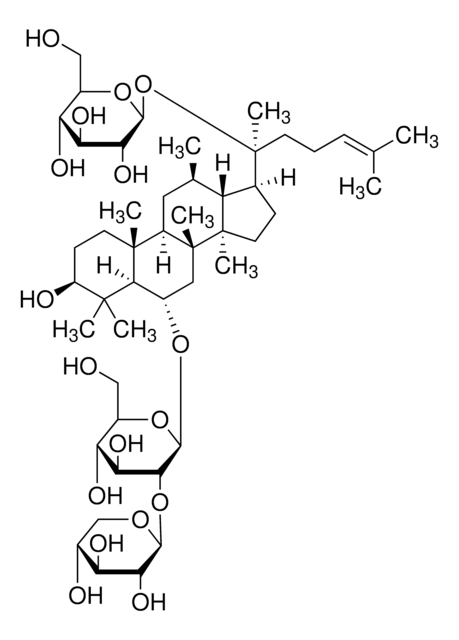
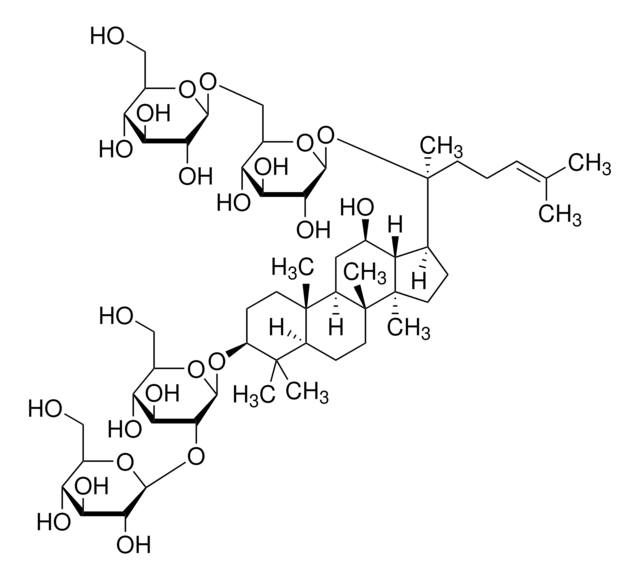
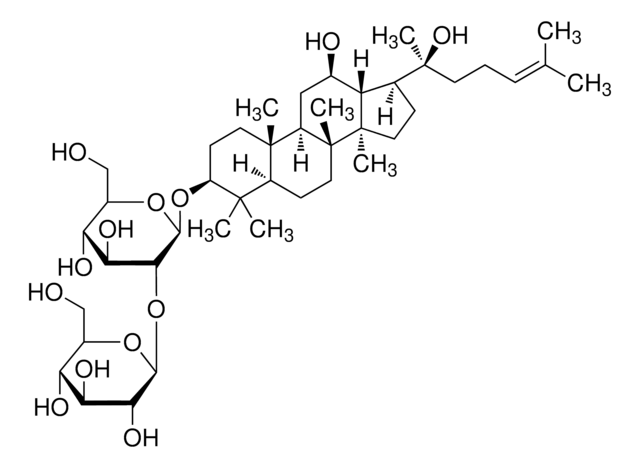
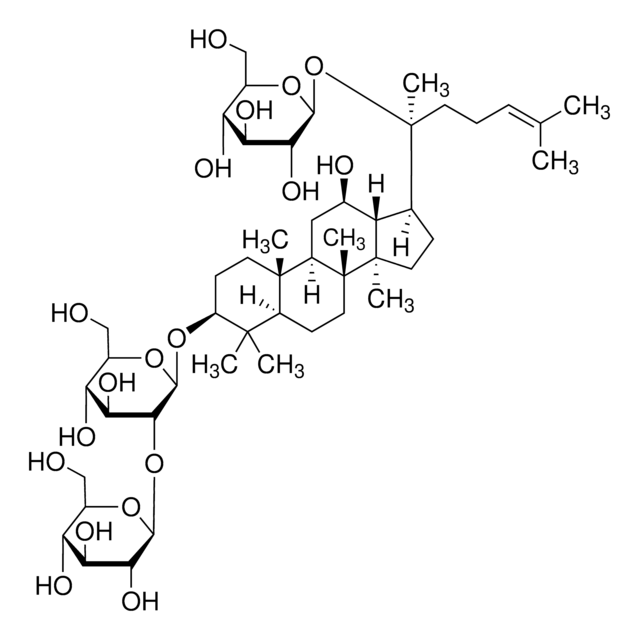
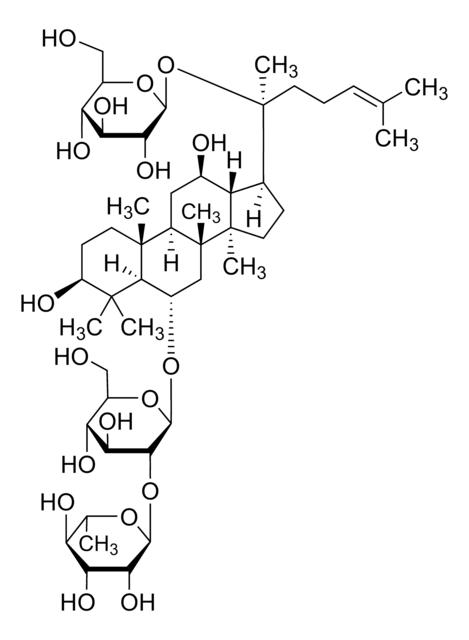
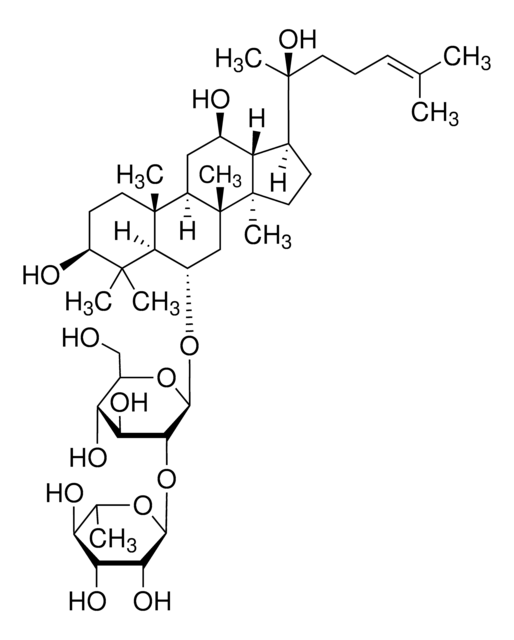
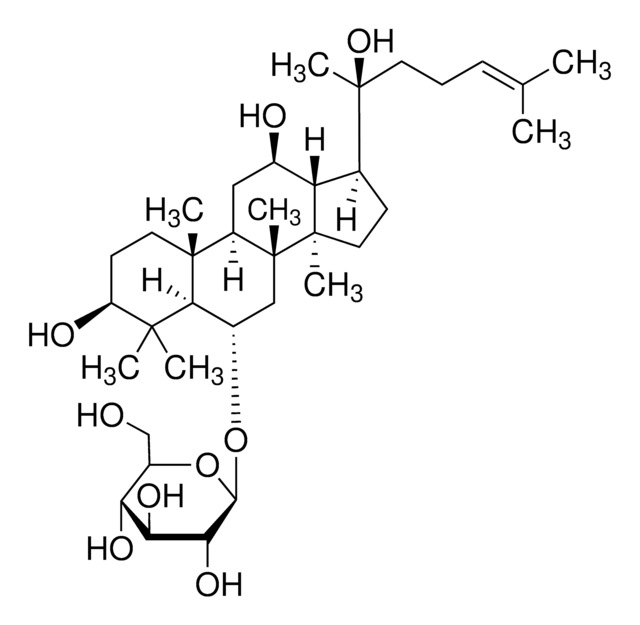
Ginsenoside Rg1
primary reference standard
동의어(들):
(3β,6α,12β)-3,12-Dihydroxydammar-24-ene-6,20-diyl bis-β-D-glucopyranoside, Ginsenoside A2, Ginsenoside g1, Panaxoside A, Panaxoside Rg1, Sanchinoside C1, Sanchinoside Rg1
About This Item
추천 제품
Grade
primary reference standard
유통기한
limited shelf life, expiry date on the label
제조업체/상표
HWI
기술
HPLC: suitable
gas chromatography (GC): suitable
배송 상태
dry ice
저장 온도
−20°C
SMILES string
C[C@]12[C@@](C[C@H]([C@@]3([H])[C@]2(CC[C@]3([H])[C@@](CCC=C(C)C)(C)O[C@@H]4O[C@@H]([C@H]([C@@H]([C@H]4O)O)O)CO)C)O)([H])[C@@]5([C@@](C(C)([C@H](CC5)O)C)([H])[C@H](C1)O[C@@H]6O[C@@H]([C@H]([C@@H]([C@H]6O)O)O)CO)C
InChI
1S/C42H72O14/c1-20(2)10-9-13-42(8,56-37-34(52)32(50)30(48)25(19-44)55-37)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24+,25+,26+,27-,28-,29+,30+,31-,32-,33+,34+,35-,36+,37-,39+,40+,41+,42-/m0/s1
InChI key
YURJSTAIMNSZAE-HHNZYBFYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Exact content by quantitative NMR can be found on the certificate.
애플리케이션
기타 정보
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
Ginsenosides Separation in Ginseng. The HPLC method was first optimized using a ginsenoside standard mixture, and was then applied to a sample of American Ginseng root.
In this article we present several HPTLC applications and analytical standards for ginsenosides.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.