07-2980
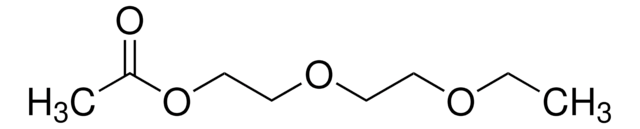
Diethylene glycol monoethyl ether
SAJ first grade, ≥98.0%
동의어(들):
2-(2-Ethoxyethoxy)ethanol, CARBITOL™, Diethylene glycol ethyl ether, Ethyldiglycol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C2H5OCH2CH2OCH2CH2OH
CAS Number:
Molecular Weight:
134.17
Beilstein:
1736441
EC Number:
MDL number:
UNSPSC 코드:
12352112
PubChem Substance ID:
추천 제품
Grade
SAJ first grade
vapor density
4.63 (vs air)
vapor pressure
0.12 mmHg ( 20 °C)
분석
≥98.0%
양식
liquid
expl. lim.
1.2 %, 135 °F
23.5 %, 182 °F
재고 정보
available only in Japan
refractive index
n20/D 1.427 (lit.)
bp
202 °C (lit.)
density
0.999 g/mL at 25 °C (lit.)
SMILES string
CCOCCOCCO
InChI
1S/C6H14O3/c1-2-8-5-6-9-4-3-7/h7H,2-6H2,1H3
InChI key
XXJWXESWEXIICW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
법적 정보
CARBITOL is a trademark of The Dow Chemical Company or an affiliated company of Dow
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
204.8 °F - closed cup
Flash Point (°C)
96 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Yousef Javadzadeh et al.
Colloids and surfaces. B, Biointerfaces, 82(2), 422-426 (2010-10-19)
Regarding the potential severe toxicity associated with systemic administration of methotrexate (MTX), a topical formulation might be of greater utility for the treatment of psoriasis and other hyperproliferative skin disorders. One of the presumed reasons for the lack of clinical
Liandong Hu et al.
International journal of pharmaceutics, 420(2), 251-255 (2011-09-13)
The objective of the present study was to formulate a microemulsion system for oral administration to improve the solubility and bioavailability of fenofibrate. Various formulations were prepared using different ratios of oils, surfactants and co-surfactants (S&CoS). Pseudo-ternary phase diagrams were
Dong Hoon Oh et al.
International journal of pharmaceutics, 420(2), 412-418 (2011-09-29)
In order to compare the effects of hydrophilic and hydrophobic solid carrier on the formation of solid self-microemulsifying drug delivery system (SMEDDS), two solid SMEDDS formulations were prepared by spray-drying the solutions containing liquid SMEDDS and solid carriers. Colloidal silica
Hyun-Jong Cho et al.
International journal of pharmaceutics, 423(2), 153-160 (2012-01-03)
To achieve rapid onset of action and improved bioavailability of udenafil, a microemulsion system was developed for its intranasal delivery. Phase behavior, particle size, transmission electron microscope (TEM) images, and the drug solubilization capacity of the microemulsion were investigated. A
Roberta Censi et al.
Drug development and industrial pharmacy, 38(9), 1128-1133 (2011-12-23)
A microemulsion for the cutaneous release of quercetin was prepared. An aqueous phase, containing 40% Transcutol® P as solubilizing agent and permeation enhancer, was emulsified with Labrafil® as oil phase and Labrasol®/Capryol™ 90 as Solvent/Co-solvent. Quercetin was dissolved in the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.