8.03779
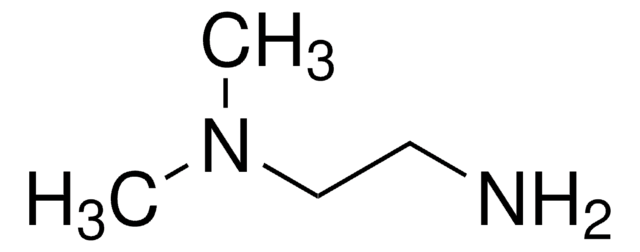
N,N-Dimethylethylenediamine
for synthesis
동의어(들):
N,N-Dimethylethylenediamine, 2-(Dimethylamino)ethylamine, 1-Amino-2-dimethylaminoethane
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
NH2C2H4N(CH3)2
CAS Number:
Molecular Weight:
88.15
MDL number:
UNSPSC 코드:
12352116
EC 인덱스 번호:
203-541-2
NACRES:
NA.22
추천 제품
vapor pressure
29 hPa ( 20 °C)
Quality Level
분석
≥98.0% (GC)
autoignition temp.
225 °C
효능
1135 mg/kg LD50, oral (Rat)
expl. lim.
1.3-10.8 % (v/v)
pH
11.4 (20 °C, 10 g/L in H2O)
bp
107-109 °C/1013 hPa
mp
<-70 °C
전이 온도
flash point 12.5 °C
density
0.81 g/cm3 at 20 °C
저장 온도
2-30°C
SMILES string
N(CCN)(C)C
InChI
1S/C4H12N2/c1-6(2)4-3-5/h3-5H2,1-2H3
InChI key
DILRJUIACXKSQE-UHFFFAOYSA-N
일반 설명
N,N-Dimethylethylenediamine (DMEDA) is used as a bidentate ligand in coordination chemistry due to the presence of two nitrogen atoms, one with lone pairs that can coordinate to a metal center. It is used as a ligand and base in Suzuki-Miyaura and Sonogashira coupling reactions, enhancing palladium catalyst stability, and deprotonating alkynes. It acts as a nucleophile and a base, promoting reactions involving proton transfer or nucleophilic attack. It is commonly used in amination reactions to synthesize substituted amines and in epoxide ring openings to form amino alcohols. Additionally, DMEDA serves as a chelating agent, forming stable complexes with transition metals. It acts as a secondary amine in reductive amination to synthesize tertiary amines.
애플리케이션
- N, N′, N′′ versus N, N′, O imine-containing coordination motifs: ligand-directed synthesis of mononuclear and binuclear CuII compounds: This study explores ligand-directed synthesis involving N,N-dimethylethylenediamine in copper compounds (RE Ferraz de Paiva et al., 2017).
- Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation: Research on the dehydrogenative condensation of methanol and N,N-dimethylethylenediamine for hydrogen storage (Z Shao et al., 2020).
- Influence of water vapor and acid gases on CO2 adsorption using N,N-dimethylethylenediamine decorated Cu-BTC: This article investigates the effects of environmental factors on the adsorption efficiency of Cu-BTC decorated with N,N-dimethylethylenediamine (F Song et al., 2019).
- Triple chemical derivatization strategy assisted liquid chromatography-mass spectrometry for determination of retinoic acids in human serum: A study on the chemical derivatization involving N,N-dimethylethylenediamine for analyzing retinoic acids in serum (GG Gong et al., 2022).
- Silicoaluminophosphate molecular sieve DNL-6: Synthesis with a novel template, N, N′-dimethylethylenediamine, and its catalytic application: Discusses the synthesis and catalytic applications of a molecular sieve developed using N,N′-dimethylethylenediamine as a template (P Wu et al., 2018).
분석 메모
Assay (GC, area%): ≥ 98.0 % (a/a)
Density (d 20 °C/ 4 °C): 0.806 - 0.809
Identity (IR): passes test
Density (d 20 °C/ 4 °C): 0.806 - 0.809
Identity (IR): passes test
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
53.1 °F - closed cup
Flash Point (°C)
11.7 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.