688000
Y-27632
≥95% (HPLC), solid, Rho kinase inhibitor, Calbiochem
동의어(들):
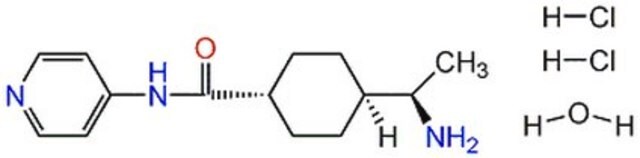
Y-27632, (R)-(+)-trans-N-(4-Pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide, 2HCl, ROCK Inhibitor, Rho Kinase Inhibitor VI, ROCK Inhibitor, (R)-(+)-trans-N-(4-Pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide, 2HCl, Rho Kinase Inhibitor VI
About This Item
추천 제품
product name
Y-27632-CAS 331752-47-7-Calbiochem, Y-27632A, CAS 331752-47-7, is a cell-permeable, reversible, inhibitor of Rho kinases (Ki = 140 nM for p160ROCK). Enhances survival & cloning efficiency of ESC without affecting their pluripotency.
Quality Level
분석
≥95% (HPLC)
형태
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated (hygroscopic)
protect from light
색상
white
solubility
water: 100 mg/mL
배송 상태
ambient
저장 온도
−20°C
InChI
1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11?,12?/m1/s1
InChI key
IYOZTVGMEWJPKR-VOMCLLRMSA-N
일반 설명
생화학적/생리학적 작용
p160 Rho-associated protein kinases (ROCK)
포장
경고
재구성
기타 정보
Watanabe, K., et al. 2007. Nature Biotech.25, 681.
Chitaley, K., et al. 2001. Nat. Med.7, 119.
Davies, S.P., et al. 2000. Biochem. J. 351, 95.
Narumiya, S., et al. 2000. Methods Enzymol.325, 273.
Hirose, M., et al. 1998. J. Cell Biol.141, 1625.
Maekawa, M., et al. 1999. Science285, 895.
Uehata, M., et al. 1997. Nature389, 990.
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.