362330
GAG Antagonist, Surfen
The GAG Antagonist, Surfen controls the biological activity of GAG. This small molecule/inhibitor is primarily used for Activators/Inducers applications.
동의어(들):
GAG Antagonist, Surfen, Gβγ Activator, 12155, NSC12155, bis-2-Methyl-4-amino-quinolyl-6-carbamide, diHCl, trihydrate, Glycosaminoglycans Antagonist
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
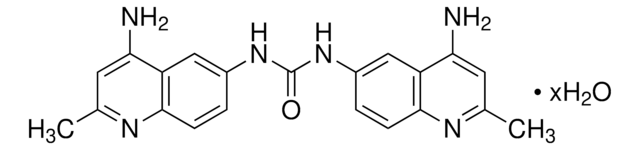
C21H20N6O · 2HCl · 3H2O
Molecular Weight:
499.39
UNSPSC 코드:
12352200
추천 제품
Quality Level
분석
≥98% (HPLC)
형태
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated (hygroscopic)
protect from light
색상
white
solubility
DMSO: 50 mg/mL
배송 상태
ambient
저장 온도
2-8°C
일반 설명
A symmetrical quinolyl-urea compound that binds GAGs (glycosaminoglycans) via electrostatic interaction with the negatively charged sulfate and carboxyl moieties present in heparan sulfate (HS), heparin, and dermatan sulfate, resulting in effective blockage of GAGs interactions with their protein binding partners. Surfen is shown to effectively neutralize HS- and heparin-mediated thrombin inhibition of Factor Xa activity as well as heparin′s anti-clotting activity. Also reported to inhibit FGF2-induced Erk phosphorylation and tubulation in murine lung endothelial cultures (IC50 ~5 µM), fibronectin HS-binding domain-dependent CHO cell adhesion (IC50 = 3 µM), and HSV-1 infection of glucosaminyl 3-O-sulfotransferase-3A-expressing CHO cells (complete inhibition at 5 µM). Surfen analogs with improved potency may serve as promising candidates as less toxic alternatives to Protamine (Cat. No. 539122) in clinical applications.Reported to directly bind Gβγ subunit in a reversible manner, displace Gα-GDP from Gβγ, and acutely activate Gβγ signaling without Gα activation.
A symmetrical quinolyl-urea compound that binds GAGs (glycosaminoglycans) via electrostatic interaction with the negatively charged sulfate and carboxyl moieties present in heparan sulfate (HS), heparin, and dermatan sulfate, resulting in effective blockage of GAGs interactions with their protein binding partners. Surfen is shown to effectively neutralize HS- and heparin-mediated thrombin inhibition of Factor Xa activity as well as heparin′s anti-clotting activity. Also reported to inhibit FGF2-induced Erk phosphorylation and tubulation in murine lung endothelial cultures (IC50 ~5 µM), fibronectin HS-binding domain-dependent CHO cell adhesion (IC50 = 3 µM), and HSV-1 infection of glucosaminyl 3-O-sulfotransferase-3A-expressing CHO cells (complete inhibition at 5 µM). Surfen analogs with improved potency may serve as promising candidates as less toxic alternatives to Protamine (Cat. No. 539122) in clinical applications.
포장
Packaged under inert gas
경고
Toxicity: Standard Handling (A)
재구성
Following reconstitution, aliquot into glass vials and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
기타 정보
Surve, C.R., et al. 2016. Sci. Signal.9, ra22.
Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA105, 13074.
Hunter, D.T. Jr., and Hill, J.M. 1961. Nature191, 1378.
Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA105, 13074.
Hunter, D.T. Jr., and Hill, J.M. 1961. Nature191, 1378.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Ferenc Zsila
Biochemical and biophysical research communications, 460(3), 863-867 (2015-04-02)
It is shown that the heparin antagonist bis-aminoquinoline derivative surfen interacts with sulfated cyclodextrins in a unique fashion. Analysis of the UV spectroscopic data revealed exceptionally strong association (K(a) ∼ 10(7) M(-1)) of several surfen molecules to the external surface
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.