추천 제품
생물학적 소스
Serratia marcescens
Quality Level
재조합
expressed in E. coli
설명
suitable for biopharmaceutical production EMPROVE® bio
제품 라인
EMPROVE® EXPERT
분석
≥99% (SDS-PAGE)
양식
buffered aqueous glycerol solution
농도
≥250 units/μL
pH
8.0 (25 °C in H2O)
응용 분야
pharma/biopharma processes
배송 상태
dry ice
저장 온도
-10 to -25°C
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
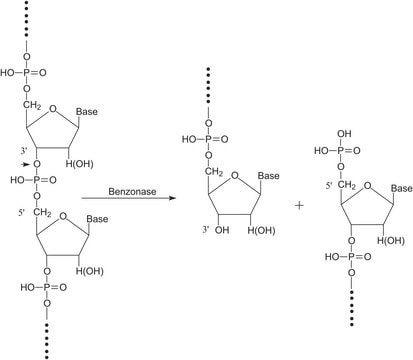
Benzonase® endonuclease acts as an unspecific endonuclease, degrading both DNA and RNA to small 3–5 base pairs (< 6 kDa) fragments with no base preference. It is the ideal tool for nucleic acid removal in virus vector and vaccine manufacturing as demanded by regulators. Additionally, the use of Benzonase® endonuclease increases the yield in virus purification, protects downstream chromatography and filter devices from fouling, and reduces feed stream viscosity.
Benzonase® endonuclease is the smart solution for DNA removal in viral vaccine and viral vector production and has proven its value for over 30 years. Balancing efficiency and regulatory compliance by delivering reliability and high-quality due manufacturing under good manufacturing practices (GMP ICH Q7). Our extensive documentation packages of Emprove® dossiers and the availability of a FDA Drug Master File type II, supports you in filing your vaccine manufacturing process to the authorities.
Benzonase® endonuclease is the smart solution for DNA removal in viral vaccine and viral vector production and has proven its value for over 30 years. Balancing efficiency and regulatory compliance by delivering reliability and high-quality due manufacturing under good manufacturing practices (GMP ICH Q7). Our extensive documentation packages of Emprove® dossiers and the availability of a FDA Drug Master File type II, supports you in filing your vaccine manufacturing process to the authorities.
Our SAFC® portfolio of high-quality products for biopharmaceutical processing withstands strict quality control procedures and is produced according to MQ-500 requirements as defined by the M-Clarity program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
애플리케이션
Used for the removal of nucleic acid from protein samples.
생화학적/생리학적 작용
Digests native or heat-denatured DNA and RNA.
분석 메모
Appearance (description): clear
Appearance (color): colourless
Activity (DNA; pH 8.0; 30 min; 37 °C): ≥ 250 U/µl
Spec. activity (calc. on protein): ≥ 1.1E+06 U/mg
Identity (electrophoresis): passes test
Purity (calc. on protein): ≥ 99.0%
Proteases: not detectable
Colony count (aerobic bacteria): <10 CFU in 100 000 U
Colony count (Yeasts and moulds): <10 CFU in 100 000 U
Endotoxines (LAL-test): <0.25 EU in 1000 U
Appearance (color): colourless
Activity (DNA; pH 8.0; 30 min; 37 °C): ≥ 250 U/µl
Spec. activity (calc. on protein): ≥ 1.1E+06 U/mg
Identity (electrophoresis): passes test
Purity (calc. on protein): ≥ 99.0%
Proteases: not detectable
Colony count (aerobic bacteria): <10 CFU in 100 000 U
Colony count (Yeasts and moulds): <10 CFU in 100 000 U
Endotoxines (LAL-test): <0.25 EU in 1000 U
법적 정보
Benzonase is a registered trademark of Merck KGaA, Darmstadt, Germany
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFC is a registered trademark of Merck KGaA, Darmstadt, Germany
또한 이 제품과 함께 일반적으로 구입
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.