W372307
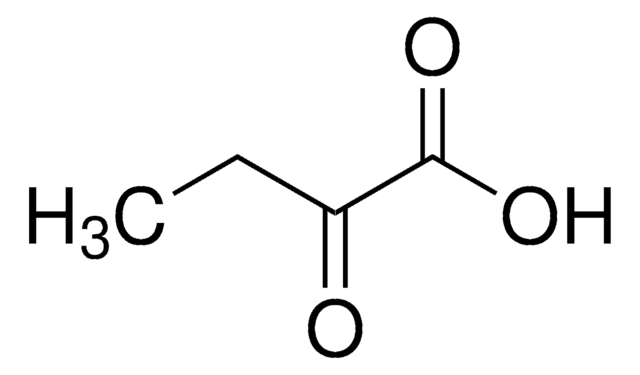
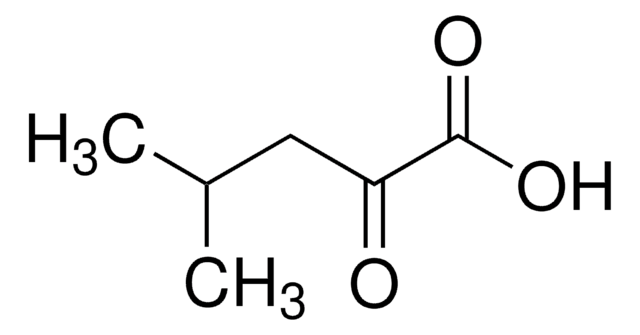
2-Oxobutyric acid
≥95%, FG
동의어(들):
2-Ketobutyric acid, α-Ketobutyric acid, Propionylformic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH3CH2COCOOH
CAS Number:
Molecular Weight:
102.09
FEMA Number:
3723
Beilstein:
1700514
EC Number:
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
8.066
NACRES:
NA.21
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Fragrance grade
Kosher
Agency
follows IFRA guidelines
규정 준수
EU Regulation 1223/2009
EU Regulation 1334/2008 & 872/2012
분석
≥95%
bp
84 °C/20 mmHg (lit.)
mp
30-34 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
향수 알레르기항원
no known allergens
감각 수용성의
caramel; creamy; brown; sweet
저장 온도
2-8°C
SMILES string
CCC(=O)C(O)=O
InChI
1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7)
InChI key
TYEYBOSBBBHJIV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
2-Oxobutyric acid is mainly found in the hydrolysates of proteins, reportedly being formed by the degradation of threonine.
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
179.6 °F - closed cup
Flash Point (°C)
82 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Review of thermally produced imitation meat flavors.
Wilson RA
Journal of Agricultural and Food Chemistry, 23(6), 1032-1037 (1975)
A Probable Flavoring Principle in Vegetable?Protein Hydrolysates.
Sulser H, et al.
Journal of Food Science, 32(6), 611-615 (1967)
K Yaegaki et al.
Journal of periodontology, 63(9), 783-789 (1992-09-01)
The amounts of volatile sulfur compounds (VSC) and methyl mercaptan/hydrogen sulfide ratio in mouth air from patients with periodontal involvement were 8 times greater than those of control subjects. Our studies demonstrated that, in patients with periodontal disease: 1) the
Sergey V Smirnov et al.
FEMS microbiology letters, 273(1), 70-77 (2007-06-15)
A two-step enzymatic synthesis process of 4-hydroxyisoleucine is suggested. In the first step, the aldol condensation of acetaldehyde and alpha-ketobutyrate catalyzed by specific aldolase results in the formation of 4-hydroxy-3-methyl-2-keto-pentanoate (HMKP). In the second step, amination of HMKP by the
Asymmetric formal [3+2] cycloaddition reaction of isocyanoesters to 2-oxobutenoate esters by a multifunctional chiral silver catalyst.
Jin Song et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7786-7790 (2011-05-28)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.