모든 사진(1)
About This Item
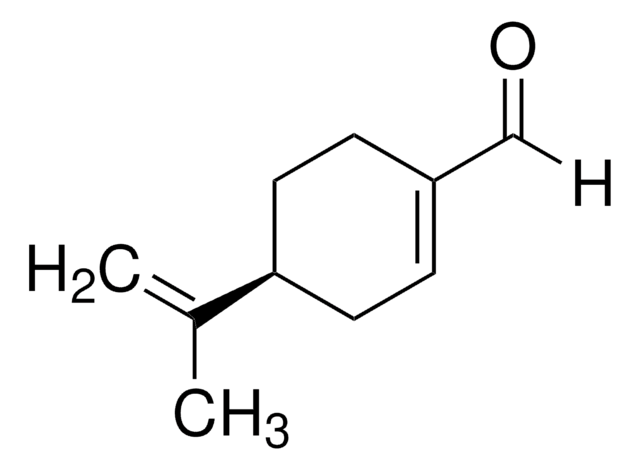
실험식(Hill 표기법):
C10H16O
CAS Number:
Molecular Weight:
152.23
FEMA Number:
2664
유럽평의회 번호:
2024
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
2.060
NACRES:
NA.21
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Halal
Kosher
규정 준수
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
분석
≥95%
광학 활성
[α]20/D −88°, c = 1 in methanol
refractive index
n20/D 1.501 (lit.)
bp
119-121 °C/11 mmHg (lit.)
density
0.96 g/mL at 25 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
감각 수용성의
fatty; green
SMILES string
CC(=C)[C@H]1CCC(CO)=CC1
InChI
1S/C10H16O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,10-11H,1,4-7H2,2H3/t10-/m1/s1
InChI key
NDTYTMIUWGWIMO-SNVBAGLBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- CYP108N12 initiates p-cymene biodegradation in Rhodococcus globerulus.: This study explores the enzymatic breakdown pathways of monoterpenes, using (S)-(−)-Perillyl alcohol as a precursor, offering insights into microbial degradation processes that could be vital for bioremediation efforts or synthetic biology applications (Giang et al., 2022).
- Orofacial antinociceptive effects of perillyl alcohol associated with codeine and its possible modes of action.: Research demonstrates the pain-relieving properties of (S)-(−)-Perillyl alcohol when combined with codeine, highlighting its potential for developing new analgesic formulations in dental and facial pain management (Limeira et al., 2022).
- Orofacial antinociceptive activity of (S)-(-)-perillyl alcohol in mice: a randomized, controlled and triple-blind study.: This study underpins the effectiveness of (S)-(−)-Perillyl alcohol in reducing orofacial pain in a controlled experimental setup, providing a basis for further clinical trials in pain management (Tomaz-Morais et al., 2017).
- In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol.: Highlights the anti-tumor properties of a novel derivative of (S)-(−)-Perillyl alcohol, suggesting its potential as a therapeutic agent in oncology, with comprehensive studies on its efficacy and safety (Andrade et al., 2016).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Donghak Kim et al.
Biotechnology letters, 31(9), 1427-1431 (2009-05-22)
The catalytic turnover of cytochrome P450( cam ) from Pseudomonas putida requires two auxiliary reduction partners, putidaredoxin (Pd) and putidaredoxin reductase (PdR). We report the functional expression in Escherichia coli of tricistronic constructs consisting of P450( cam ) encoded by
Nonmelanoma skin cancer chemoprevention.
Renata Prado et al.
Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.], 37(11), 1566-1578 (2011-09-08)
Juliana de Saldanha da Gama Fischer et al.
Journal of experimental therapeutics & oncology, 7(4), 285-290 (2009-02-21)
Perillyl alcohol (POH) is a naturally occurring monoterpene with antiangiogenic and anti-tumoral properties. This chemotherapeutic agent has proven effectiveness in several clinical trials, including an ongoing phase I, comprising patients with recurrent glioblastoma multiform (GBM) under treatment with POH by
Tabetha Sundin et al.
Molecular and cellular biochemistry, 375(1-2), 97-104 (2013-01-04)
We previously demonstrated in prostate cancer cells that a phytochemical-perillyl alcohol-and the mechanistic target of rapamycin (mTOR) inhibitor rapamycin rapidly attenuated telomerase activity. Protein levels of the telomerase catalytic subunit reverse transcriptase (hTERT) were diminished in the absence of an
Sjef Cornelissen et al.
Journal of industrial microbiology & biotechnology, 38(9), 1359-1370 (2011-05-12)
Cell physiology is a critical factor determining the efficiency of reactions performed by microbial biocatalysts. In order to develop an efficient biotransformation procedure for the hydroxylation of (S)-limonene to (S)-perillyl alcohol by recombinant Pseudomonas putida cells harboring the cytochrome P450
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W266418-1KG | |
| W266418-1KG-K | 4061837800429 |
| W266418-5KG | |
| W266418-100G | |
| W266418-100G-K | 4061837800412 |
| W266418-5KG-K | 4061836712150 |
| W266418-SAMPLE | |
| W266418-SAMPLE-K | 4061837800436 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.