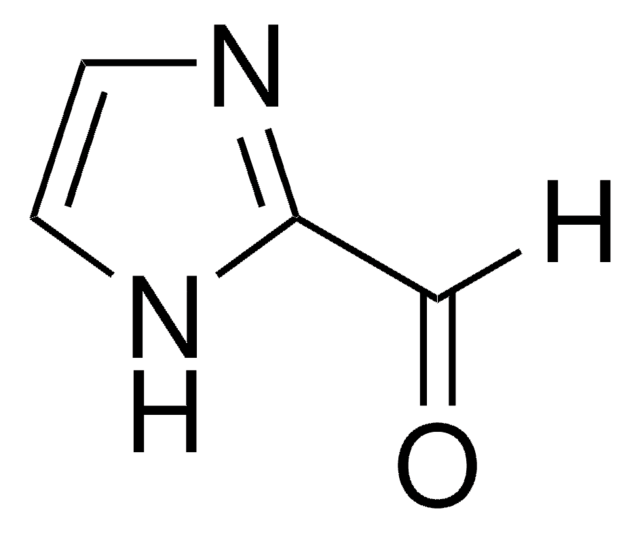
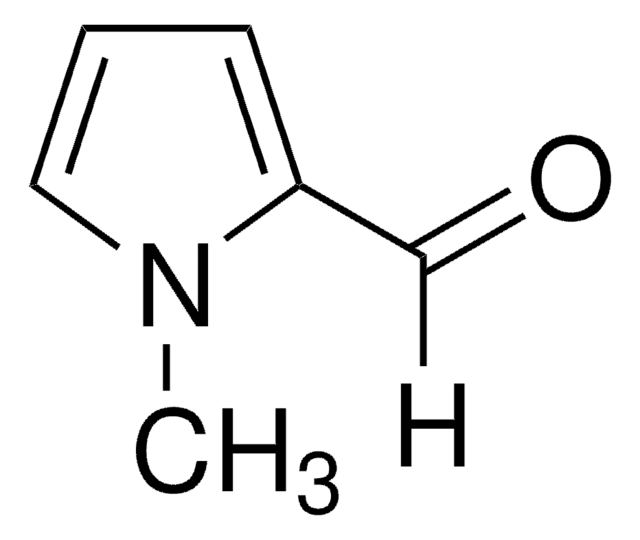
추천 제품
Quality Level
분석
98%
형태
crystals
bp
217-219 °C (lit.)
mp
43-46 °C (lit.)
저장 온도
2-8°C
SMILES string
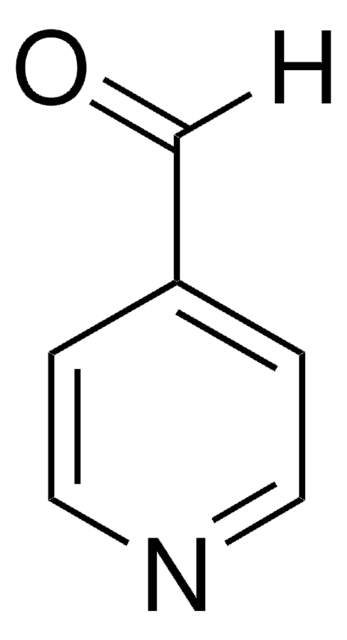
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
InChI key
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pyrrole-2-carboxaldehydesis a heterocyclic building blocks characterized by a pyrrole ring with a formylgroup attached at the 2-position used in the production of various biologicallyactive compounds. Highly functionalized pyrrole-2-carboxaldehydes have beenutilized as an intermediate in the creation of oligopyrrole macrocycles.
애플리케이션
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
224.6 °F - closed cup
Flash Point (°C)
107 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Yasushi Hikida et al.
Nature protocols, 5(7), 1312-1323 (2010-07-03)
Methods for fluorescent probing at a defined position of RNA provide powerful tools for analyzing the local structural conformation of functional RNA molecules by tracking fluorescence changes. In this article, we describe the site-specific fluorescent probing of RNA by transcription
Ryuya Fukunaga et al.
Biochemical and biophysical research communications, 372(3), 480-485 (2008-05-28)
An unnatural base pair between 7-(2-thienyl)-imidazo[4,5-b]pyridine (Ds) and pyrrole-2-carbaldehyde (Pa) could expand the genetic alphabet and allow the incorporation of non-standard amino acids into proteins at defined positions. For this purpose, we synthesized tRNAs bearing Pa at the anticodon and
Tsuneo Mitsui et al.
Journal of the American Chemical Society, 125(18), 5298-5307 (2003-05-02)
An unnatural hydrophobic base, pyrrole-2-carbaldehyde (denoted as Pa), was developed as a specific pairing partner of 9-methylimidazo[(4,5)-b]pyridine (Q). The Q base is known to pair with 2,4-difluorotoluene (F) as an isostere of the A-T pair, and F also pairs with
Aaron R Coffin et al.
The Journal of organic chemistry, 71(17), 6678-6681 (2006-08-12)
A regiocontrolled synthesis of 3,4-disubstituted pyrrole-2-carboxaldehydes was completed in two steps from acyclic starting materials. A Barton-Zard pyrrole synthesis between N-methoxy-N-methyl-2-isocyanoacetamide and alpha-nitroalkenes or beta-nitroacetates provided N-methoxy-N-methyl pyrrole-2-carboxamides (pyrrole Weinreb amides), which were converted into the corresponding pyrrole-2-carboxaldehydes by treatment
Barbara Michela Giuliano et al.
The journal of physical chemistry. A, 114(7), 2506-2517 (2010-01-30)
Monomeric pyrrole-2-carbaldehyde (P2C) was isolated in low-temperature argon and xenon matrices, and its UV-induced photochemistry was studied. The structures of the reagent as well as the reaction photoproducts were characterized by FTIR spectroscopy. Interpretation of the experimental results was assisted
프로토콜
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.