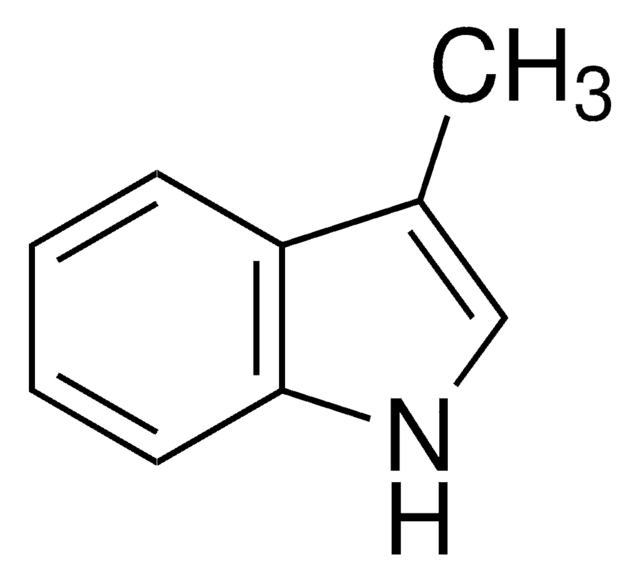
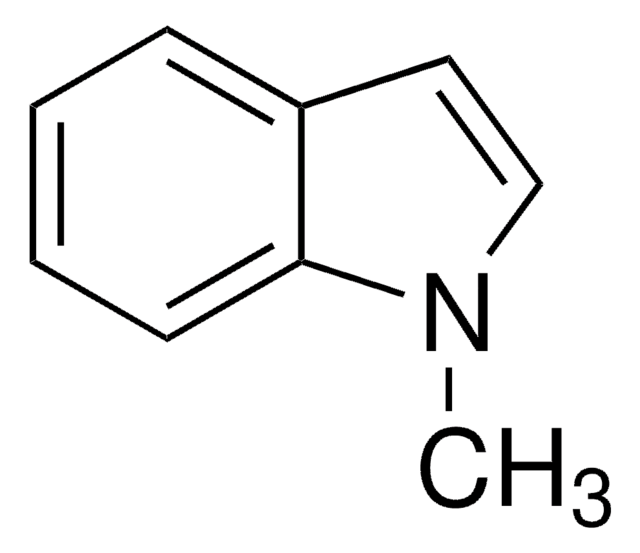
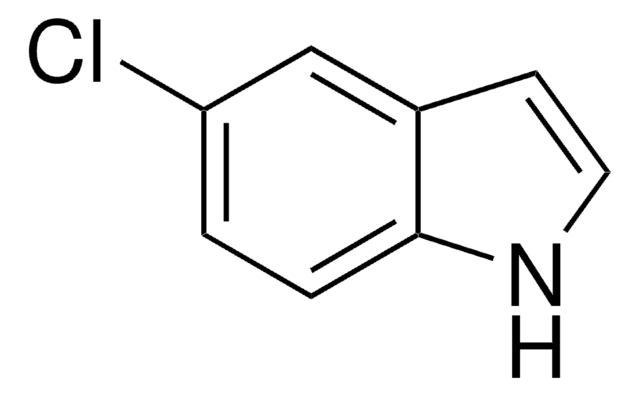
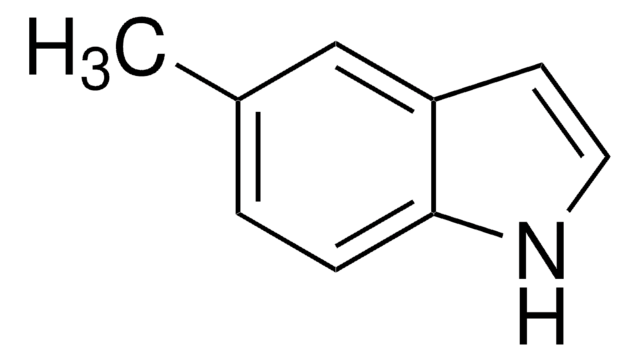
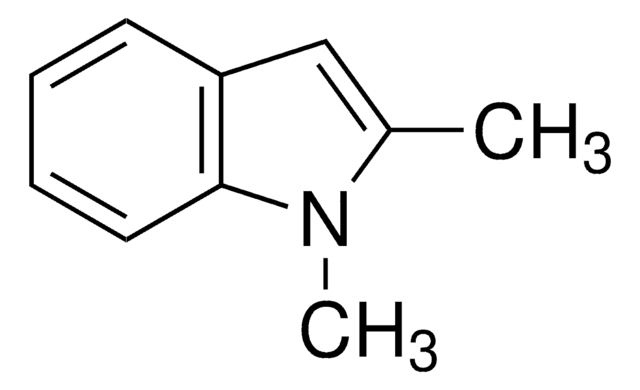
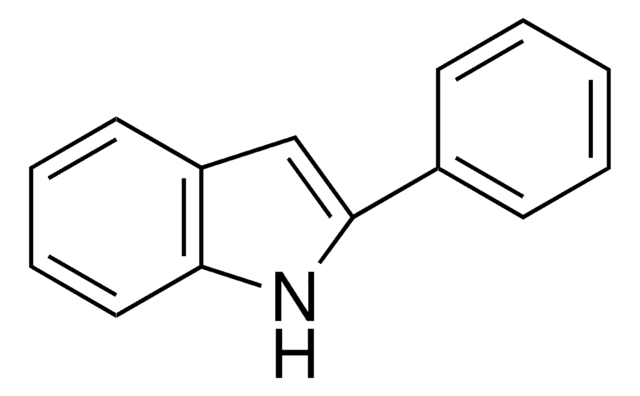
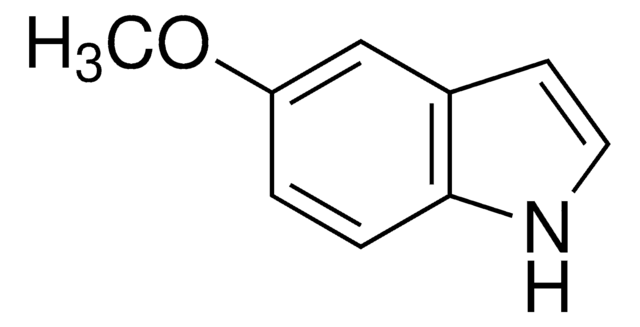
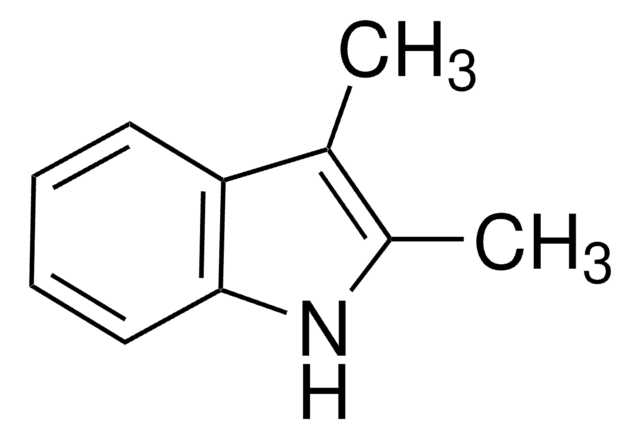
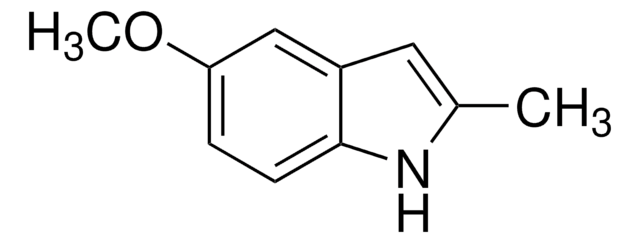
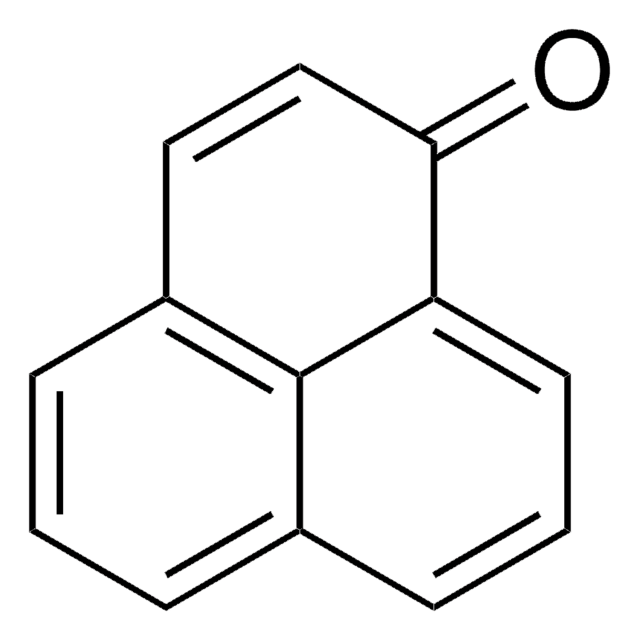
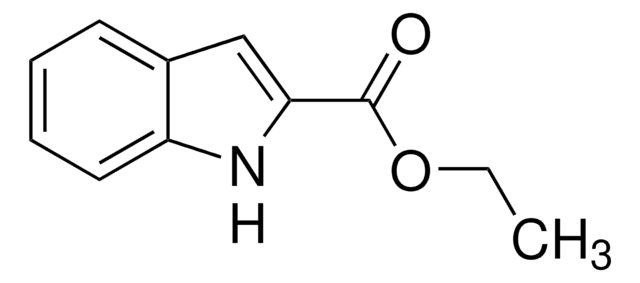
추천 제품
애플리케이션
Reactant for:
- Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
- Friedel-Crafts alkylation reactions
- Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Preparation of plant-growth inhibitors
- Michael addition reactions
- Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
285.8 °F
Flash Point (°C)
141 °C
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(8-9), 1957-1966 (2004-07-14)
Electrochemical measurements by cyclic voltammetry predict the possibility of occurrence of photoinduced electron-transfer (PET) reactions between the ground state of 2-phenylindole (2PI) (electron donor) and the excited singlet of 9-cyanoanthracene (9CNA) molecule acting as an electron acceptor. However, 2PI should
N G Faleev et al.
Biochemistry and molecular biology international, 35(5), 1037-1040 (1995-04-01)
Tryptophanase was generally considered to be inactive towards tryptophan derivatives substituted at 2-position of the indole ring. We have shown that cells containing tryptophanase catalyze the formation of 2-methyl-L-tryptophan from 2-methylindole and L-serine, and from 2-methylindole, pyruvate and ammonium ion.
Emma L Harry et al.
The Analyst, 136(8), 1728-1732 (2011-02-26)
The potential of ion mobility (IM) spectrometry in combination with mass spectrometry (MS) for real-time reaction monitoring is reported. The combined IM-MS approach using electrospray ionization affords gas-phase analyte characterization based on both mass-to-charge (m/z) ratio and gas-phase ion mobility
T Misra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(8), 1631-1641 (2002-08-09)
By using steady state and time-resolved (laser flash photolysis and single photon counting) spectroscopic techniques the quenching of the lowest excited singlet (S1) state of 9-cyanoanthracene (9CNA) by the donors (quenchers) 2-methylindole (2MI) and 2-methylindoline (2MIN) in solvents of different
[2-Methylindoles substituted in the 1st, 3d and 5th positions and the diffuse neuroendocrine APUD system].
K S Shadurskiĭ et al.
Farmakologiia i toksikologiia, 46(2), 115-120 (1983-03-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.