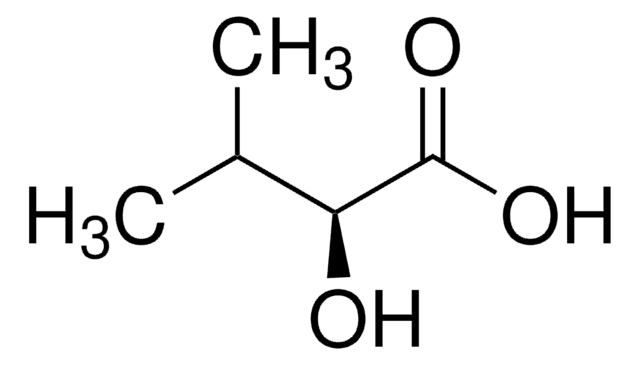
H40009
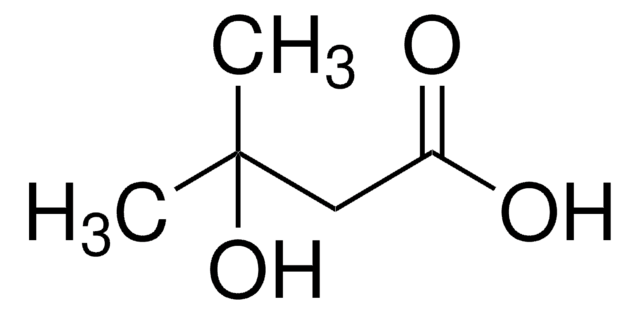
2-Hydroxy-2-methylbutyric acid
98%
동의어(들):
α-Hydroxy-α-methylbutyric acid, (±)-α-Hydroxy-α-methylbutyric acid, (±)-2-Hydroxy-2-methylbutanoic acid, (±)-2-Hydroxy-2-methylbutyric acid, 2-Hydroxy-2-methylbutanoic acid, 2-Methyl-2-hydroxybutyric acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C2H5C(CH3)(OH)CO2H
CAS Number:
Molecular Weight:
118.13
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
mp
73-75 °C (lit.)
SMILES string
CCC(C)(O)C(O)=O
InChI
1S/C5H10O3/c1-3-5(2,8)4(6)7/h8H,3H2,1-2H3,(H,6,7)
InChI key
MBIQENSCDNJOIY-UHFFFAOYSA-N
애플리케이션
2-Hydroxy-2-methylbutyric acid can be used as:
- A complexing agent in a new electrolytic system, applicable in the isotachophoretic lanthanide separation.
- An aldehyde surrogate to prepare pyrrolo[1,2-a]quinoxaline derivatives by reacting with 2-(1H-pyrrol-1-yl)aniline.
- A starting material to synthesize Cr(V) reagent named sodium bis(2-hydroxy-2-methylbutyrato)oxochromate(V) (Aldrich cat. no. ALD00006) applicable in the total synthesis of (−)-taxuyunnanine D.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Separation and analysis of lanthanides by isotachophoresis coupled with inductively coupled plasma mass spectrometry
Vio L, et al.
Talanta, 99, 586-593 (2012)
Two-phase synthesis of (−)-taxuyunnanine D
Wilde NC, et al.
Journal of the American Chemical Society, 136(13), 4909-4912 (2014)
Florence Guéguen et al.
Talanta, 162, 278-284 (2016-11-14)
The high-precision isotopic characterization of actinides and fission products in nuclear samples is fundamental for various applications such as the management of spent nuclear fuel or the validation of neutronic calculation codes. However multi-elemental isotope ratio measurements by mass spectrometric
W Dekant et al.
Research report (Health Effects Institute), (102)(102), 29-71 (2001-08-16)
The biotransformation of methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE), and tert-amyl methyl ether (TAME) was studied in humans and in rats after inhalation of 4 and 40 ppm of MTBE, ETBE, and TAME, respectively, for 4 hours, and
α-Hydroxy acid as an aldehyde surrogate: metal-free synthesis of pyrrolo [1, 2-a] quinoxalines, quinazolinones, and other N-heterocycles via decarboxylative oxidative annulation reaction
Viji M, et al.
Royal Society of Chemistry Advances, 10(61), 37202-37208 (2020)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.