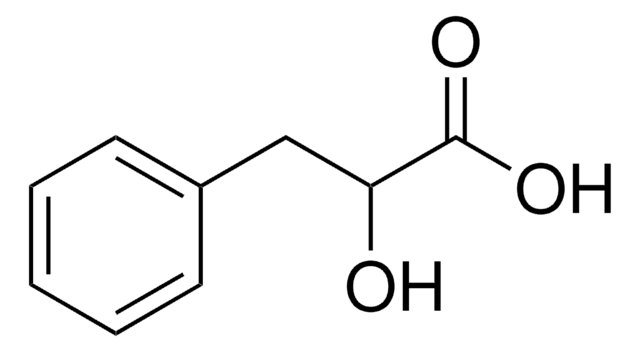
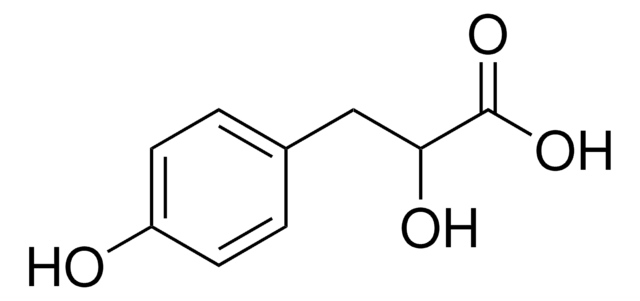
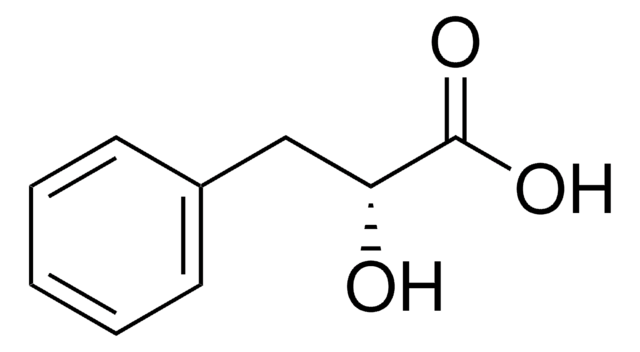
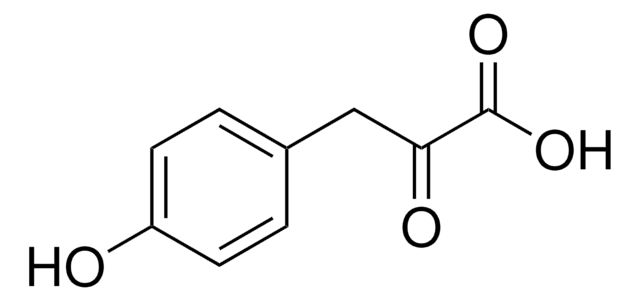
추천 제품
애플리케이션
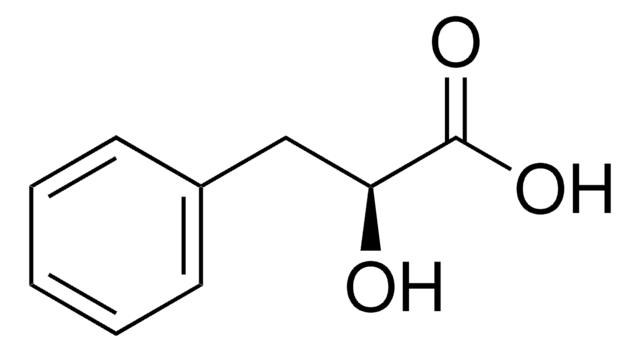
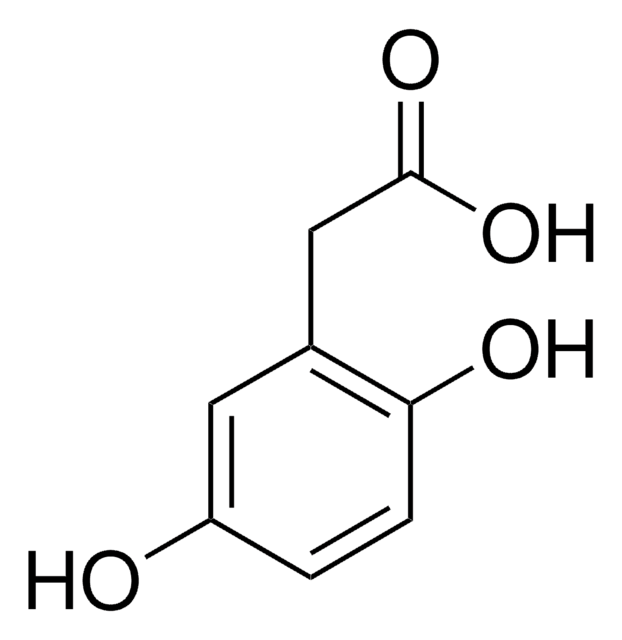
DL-p-Hydroxyphenyllactic acid is a precursor in the synthesis of rosmarinic acid that can be used for pharmaceutical and nutraceutical applications. It also exhibits antifungal activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
A M Dallagnol et al.
Journal of applied microbiology, 111(6), 1447-1455 (2011-09-29)
To evaluate the influence of biosynthetic precursors, intermediates and electron acceptors on the production of antifungal compounds [phenyllactic acid (PLA) and hydroxyphenyllactic acid (OH-PLA)] by Lactobacillus plantarum CRL 778, a strain isolated from home-made sourdough. Growth of fermentative activity and
A comprehensive investigation into sample extraction and method validation for the identification of antifungal compounds produced by lactic acid bacteria using HPLC-UV/DAD.
Brosnan B, et al.
Analytical Methods : Advancing Methods and Applications, 6(14), 5331-5344 (2014)
J C Deutsch
Journal of chromatography. B, Biomedical sciences and applications, 690(1-2), 1-6 (1997-03-07)
The synthesis and purification of [13C2]p-hydroxyphenyllactic acid from [13C2]p-hydroxyphenylpyruvic acid, the characterization of tert.-butyldimethylsilyl-derivatized tyrosine, p-hydroxyphenylpyruvic acid and p-hydroxyphenyllactic acid, and an isotope-dilution assay for these substances in normal human plasma using gas chromatography-mass spectrometry (GC-MS) are described. Using this
K B Presto Elgstoen et al.
Electrophoresis, 18(10), 1857-1860 (1998-02-12)
Capillary electrophoresis (CE) equipped with a diode-array detector have been used to determine diagnostic metabolites occurring in urine of patients with various diseases. The urine samples were injected directly onto the CE instrument without any pretreatment. Identification of abnormal metabolites
Sylvie Garneau-Tsodikova et al.
Journal of the American Chemical Society, 128(39), 12600-12601 (2006-09-28)
The red streptomycete metabolite prodigiosin has a unique tripyrrolic structure with two of the three pyrrolyl moieties in tandem. Five enzymes, PigA,G,H,I, and J, are involved in dipyrrole (rings A and B) formation. We have heterologously expressed and purified from
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.