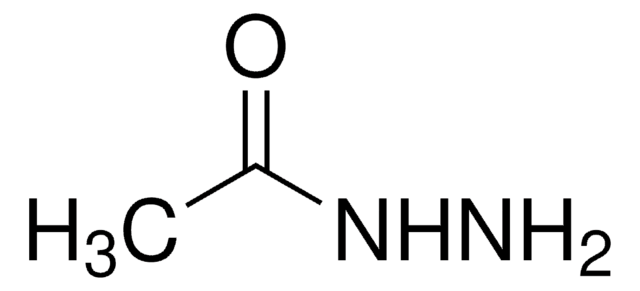
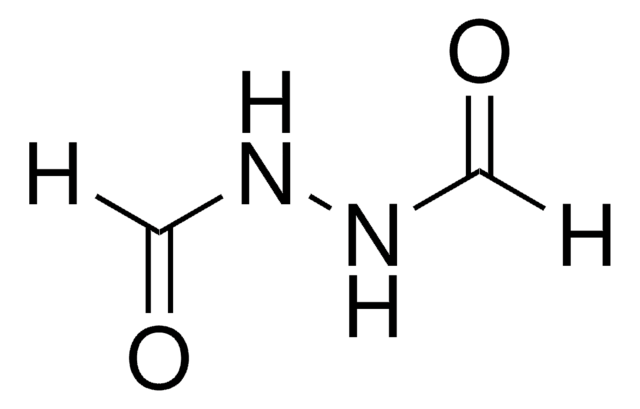
추천 제품
분석
98%
양식
powder
bp
209 °C/15 mmHg (lit.)
mp
138-140 °C (lit.)
SMILES string
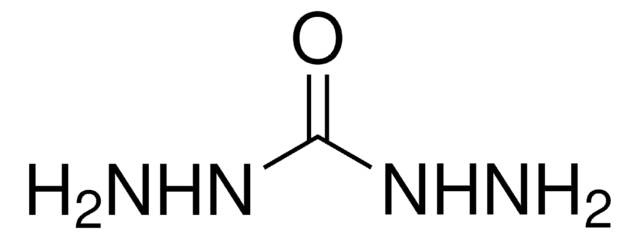
CC(=O)NNC(C)=O
InChI
1S/C4H8N2O2/c1-3(7)5-6-4(2)8/h1-2H3,(H,5,7)(H,6,8)
InChI key
ZLHNYIHIHQEHJQ-UHFFFAOYSA-N
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
E B Bhalerao et al.
Indian journal of physiology and pharmacology, 29(2), 83-88 (1985-04-01)
Patients suffering from pulmonary tuberculosis were investigated for the levels of isoniazid (INH) and its metabolites viz. acetyl-INH, mono and diacetyl hydrazines and ammonia. It was observed that 50% of the patients are slow inactivators of INH and almost all
S V Bhide et al.
Cancer letters, 23(2), 235-240 (1984-06-01)
Two hydrazine derivatives, monoacetyl hydrazine (MAH) and diacetyl hydrazine (DAH), have been tested for mutagenic response in the Salmonella/mammalian microsome assay and micronucleus test. MAH but not DAH, increased the revertant mutants in TA100 and TA1535 and also increased the
J A Timbrell et al.
Human toxicology, 3(6), 485-495 (1984-12-01)
The urinary metabolite profile of isoniazid has been studied in patients receiving the drug as therapy for tuberculosis and the profile in patients suffering liver damage due to isoniazid compared with that in control patients. There were no consistent differences
E B Bhalerao et al.
Indian journal of physiology and pharmacology, 29(3), 133-138 (1985-07-01)
Effect of isoniazid (INH) and its metabolites e.g. mono and diacetyl hydrazines (MAH and DAH respectively) was studied on circulating and tissue folates in mice (a species susceptible to INH tumorigenicity) and rats (a species resistant to INH carcinogenicity). It
Ramachandran Azhakar et al.
Dalton transactions (Cambridge, England : 2003), 41(5), 1529-1533 (2011-12-14)
The reaction of N-heterocyclic silylene (NHSi) L [L = CH{(C[double bond, length as m-dash]CH(2))(CMe)(2,6-iPr(2)C(6)H(3)N)(2)}Si] with benzoylhydrazine, 1,2-dicarbethoxyhydrazine, 1,2-diacetylhydrazine and 1,2-bis(tert-butoxycarbonyl)hydrazine in 1 : 1 molar ratio resulted in compounds 1-4 with an almost quantitative yield and five coordinate silicon atoms.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.