D7927
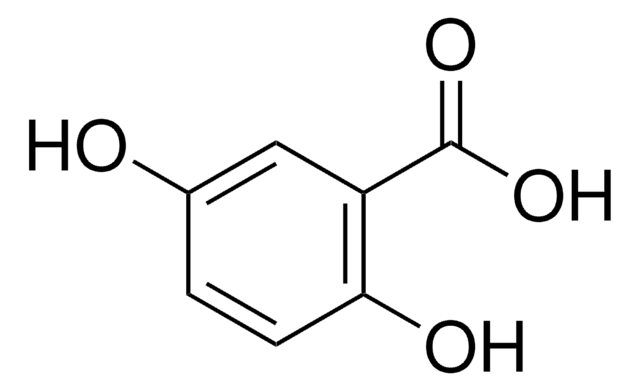
Sinapic acid
≥98%, powder
동의어(들):
3,5-Dimethoxy-4-hydroxycinnamic acid, 4-Hydroxy-3,5-dimethoxy-cinnamic acid, Sinapinic acid
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C11H12O5
CAS Number:
Molecular Weight:
224.21
Beilstein:
2699118
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥98%
형태
powder
분자량
monoisotopic mol wt 224.06839 Da
mp
~202 °C
solubility
H2O: slightly soluble (lit.)(lit.)
methanol: water: soluble (lit.)(lit.)
polar organic solvents: soluble (lit.)(lit.)
SMILES string
COc1cc(\C=C\C(O)=O)cc(OC)c1O
InChI
1S/C11H12O5/c1-15-8-5-7(3-4-10(12)13)6-9(16-2)11(8)14/h3-6,14H,1-2H3,(H,12,13)/b4-3+
InChI key
PCMORTLOPMLEFB-ONEGZZNKSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sinapic acid is an hydroxycinnamic acid derivative that occurs naturally in Brassicaceae species .
Sinapic acid is a phenylpropanoid derivative that acts as a radical scavenger with antioxidant, antimicrobial, anti-inflammatory, and anticancer properties.
Sinapic acid is a phenylpropanoid derivative that acts as a radical scavenger with antioxidant, antimicrobial, anti-inflammatory, and anticancer properties.
애플리케이션
Sinapic acid can be used:
- As a potent peroxynitrite (ONOO−) oxidant scavenger for the protection of the cellular constituents against peroxynitrite cytotoxic species.
- In the synthesis of antioxidant hydroxycinnamic acid xylan esters via esterification reaction.
- In the synthesis of pseudo-cinnamic derivatives effective against mycobacterium tuberculosis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Sinapic acid and its derivatives: natural sources and bioactivity.
Niciforovic N and Abramovivc H
Comprehensive Reviews in Food Science and Food Safety, 13(1), 34-51 (2014)
Peroxynitrite scavenging activity of sinapic acid (3, 5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea.
Zou Y, et al.
Journal of Agricultural and Food Chemistry, 50(21), 5884-5890 (2002)
Synthesis and antioxidant activity of hydroxycinnamic acid xylan esters.
Wrigstedt P, et al.
Journal of Agricultural and Food Chemistry, 58(11), 6937-6943 (2010)
Synthesis and evaluation of a novel series of pseudo-cinnamic derivatives as antituberculosis agents.
Yoya GK, et al.
Bioorganic & Medicinal Chemistry, 19(2), 341-343 (2009)
Urska Repnik et al.
The Journal of biological chemistry, 290(22), 13800-13811 (2015-04-03)
Cysteine cathepsins are primarily lysosomal proteases involved in general protein turnover, but they also have specific proteolytic functions in antigen presentation and bone remodeling. Cathepsins are most stable at acidic pH, although growing evidence indicates that they have physiologically relevant
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.