추천 제품
일반 설명
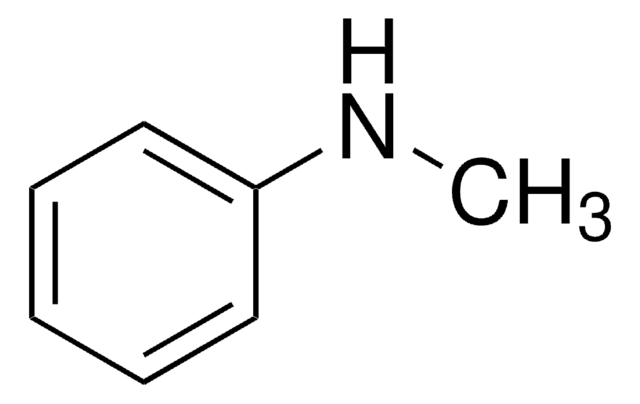
N,N-dimethylaniline (DMA) is N,N-disubstituted aniline. The thermo-physical and thermodynamic properties of the binary mixtures of DMA with anisole (ANS) and tert-butyl methyl ether (MTBE) have been reported. In acidic buffers it forms N,N,N′,N′-tetramethylbenzidine at a platinum electrode due anodic oxidation. DMA combines with diazonium salts to form methyl orange under alkaline condition. It forms ortho- and para-nitro derivatives via nitration reaction using N-bromosuccinimide/silver(I) nitrate reagent system in acetonitrile.
애플리케이션
N,N-dimethylaniline (DMA) may be used as a catalyst for reductive lithiation.
Reagent in new methodology for symmetrical and unsymmetrical photoconductor squaranes.
법적 정보
DuPont product
교체됨
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Carc. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
167.0 °F - closed cup
Flash Point (°C)
75 °C - closed cup
이미 열람한 고객
Canadian Journal of Chemistry, 71, 494-494 (1993)
Spectrophotometric determination of chromium by using sulphanilic acid and N, N-dimethylaniline.
Chandrashekhara KG, et al.
Indian Journal of Chemical Technology, 22(1-2), 78-81 (2015)
Molecular interaction study through experimental and theoretical volumetric, acoustic and refractive properties of binary liquid mixtures at several temperatures 1. N,N-dimethylaniline with aryl, and alkyl ethers.
Master ZR and Malek NI.
Journal of Molecular Liquids, 196, 120-134 (2014)
Fundamental Difference in Reductive Lithiations with Preformed Radical Anions versus Catalytic Aromatic Electron-Transfer Agents: N,N-Dimethylaniline as an Advantageous Catalyst.
Kennedy N, et al.
Angewandte Chemie (International Edition in English), 128(1), 391-394 (2016)
Aromatic nitration under neutral conditions using N-bromosuccinimide/silver (I) nitrate.
Nowrouzi N, et al.
Tetrahedron Letters, 53(36), 4841-4842 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![5-(5,6-Dimethoxy-1H-benzo[d]imidazol-2-yl)-2-methylaniline](/deepweb/assets/sigmaaldrich/product/structures/510/969/373726c0-5228-440e-8d31-b3411b331e02/640/373726c0-5228-440e-8d31-b3411b331e02.png)