모든 사진(1)
About This Item
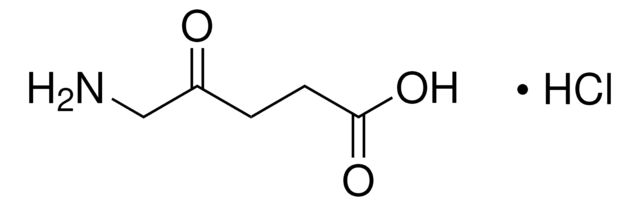
실험식(Hill 표기법):
C5H9NO3
CAS Number:
Molecular Weight:
131.13
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
추천 제품
양식
solid
SMILES string
O=C(CCCC(O)=O)N
InChI
1S/C5H9NO3/c6-4(7)2-1-3-5(8)9/h1-3H2,(H2,6,7)(H,8,9)
InChI key
GTFMAONWNTUZEW-UHFFFAOYSA-N
기타 정보
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
법적 정보
Product of ChemBridge Corp.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Cholecystokinin receptor antagonists.
K A Zucker et al.
The Journal of surgical research, 45(5), 496-504 (1988-11-01)
T Takács et al.
International journal of pancreatology : official journal of the International Association of Pancreatology, 10(1), 1-8 (1991-09-01)
In this article, the effects of different classes of cholecystokinin (CCK) receptor antagonists in CCK-related physiological processes of the pancreas have been discussed. Both glutaramic acid derivatives and natural (benzodiazepine) analogs are potent, competitive antagonists of peripheral CCK receptors. These
D J Goon et al.
Bioconjugate chemistry, 5(5), 418-422 (1994-09-01)
By use of a glutaramyl-beta-alanyl spacer group, a hapten for the polychlorinated biphenyl, 2,2',4,4',5,5'-hexachlorobiphenyl (1), viz., 2-amino-2',4,4',5,5'-pentachlorobiphenyl (2), was successfully conjugated to carrier proteins to provide immunogens with high hapten/protein molar substitution ratios (MSR's). The procedure allows for the incorporation
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.