920894
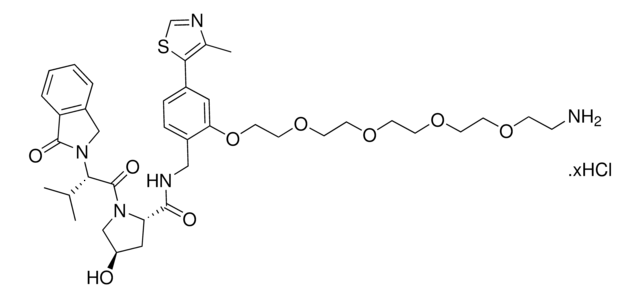
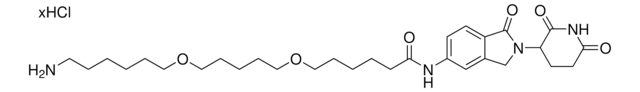
(S,R,S)-VL285 Phenol-PEG2-NH2 hydrochloride
동의어(들):
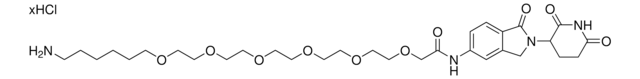
(2R,4S)-N-(2-(2-(2-(2-Aminoethoxy)ethoxy)ethoxy)-4-(4-methylthiazol-5-yl)benzyl)-4-hydroxy-1-((R)-3-methyl-2-(1-oxoisoindolin-2-yl)butanoyl)pyrrolidine-2-carboxamide hydrochloride, Crosslinker−E3 Ligase ligand conjugate, VHL protein degrader building block for PROTAC® research
About This Item
추천 제품
ligand
VL285 phenol
Quality Level
양식
solid
반응 적합성
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
작용기
amine
저장 온도
2-8°C
SMILES string
O=C([C@H]1C[C@H](O)CN1C([C@@H](C(C)C)N2CC(C=CC=C3)=C3C2=O)=O)NCC4=CC=C(C5=C(C)N=CS5)C=C4OCCOCCOCCN.Cl
InChI
1S/C35H45N5O7S.ClH/c1-22(2)31(40-19-26-6-4-5-7-28(26)34(40)43)35(44)39-20-27(41)17-29(39)33(42)37-18-25-9-8-24(32-23(3)38-21-48-32)16-30(25)47-15-14-46-13-12-45-11-10-36;/h4-9,16,21-22,27,29,31,41H,10-15,17-20,36H2,1-3H3,(H,37,42);1H/t27-,29+,31+;/m0./s1
InChI key
RJXJVEJULBIZRR-YSQLAWOQSA-N
애플리케이션
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
기타 정보
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
법적 정보
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.