추천 제품
애플리케이션
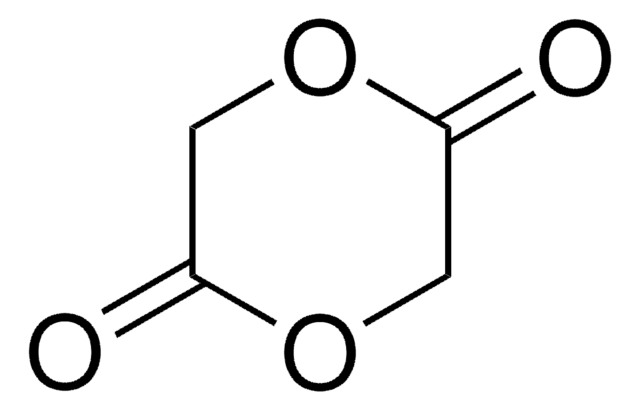
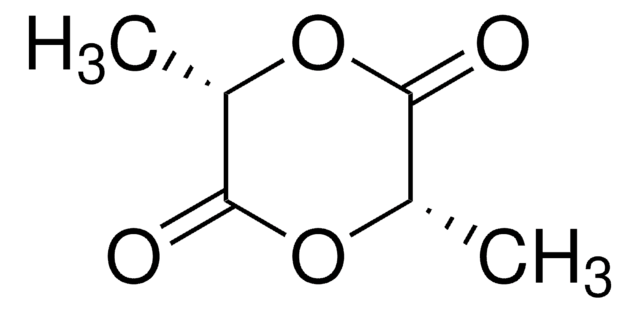
3-Methylglycolide (MG) is a six-member lactone consisting of one lactyl unit (L) and one glycolyl unit (G), it is used for the synthesis of poly (lactic-co-glycolic acid) (PLGA) polymer. Polymerization of MG follows the same mechanism as that of glycolide (GA) and Lactide (LA), and the resulting polymers possess the exact alternative sequence of glycolide (GA) and Lactide (LA). PLGA based polymers made from MG perfectly avoid the drawback of structure with long glycolic blocks, exhibit excellent solubility in common organic solvents, such as acetonitrile, acetone, dioxane, DCM and THF, thus solve the long-time headache of insolubility and discoloration issues of PLGA based polymers, providing great convenience for drug delivery researches and applications.
Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer that has been approved by the FDA for biomedical and pharmaceutical applications. PLGA based polymers can be synthesized from copolymerization of glycolide (GA) and lactide (LA), and PLGAs that comprise up to a 1:1 ratio of lactic to glycolic units are of practical interest. However, the copolymerization of glycolide (GA) and Lactide (LA) typically results in broad composition ranges and a random block nature because of the much higher reactivity of GA and the drastic polymerization conditions. Thus, simple use of equimolar charges of GA and LA results in polymers containing longer glycolic blocks. This adversely affects the solubility and PDI of the copolymer. Melt copolymerization of GA and LA has often been used to prepare PLGA with high glycolic content. Under these conditions, in situ transesterification of the polymer both randomizes the sequence and broadens the distribution, as well as significant discoloration of the resulting PLGA copolymer
Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer that has been approved by the FDA for biomedical and pharmaceutical applications. PLGA based polymers can be synthesized from copolymerization of glycolide (GA) and lactide (LA), and PLGAs that comprise up to a 1:1 ratio of lactic to glycolic units are of practical interest. However, the copolymerization of glycolide (GA) and Lactide (LA) typically results in broad composition ranges and a random block nature because of the much higher reactivity of GA and the drastic polymerization conditions. Thus, simple use of equimolar charges of GA and LA results in polymers containing longer glycolic blocks. This adversely affects the solubility and PDI of the copolymer. Melt copolymerization of GA and LA has often been used to prepare PLGA with high glycolic content. Under these conditions, in situ transesterification of the polymer both randomizes the sequence and broadens the distribution, as well as significant discoloration of the resulting PLGA copolymer
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Synthesis of O-(2′-Bromopropionyl)glycolic Acid and Its Polymerization: Synthesis of an Alternating Lactic and Glycolic Acid Copolymer.
Rebert WN
Macromolecules, 27, 5533-5535 (1994)
Lin Yu et al.
Biomacromolecules, 12(4), 1290-1297 (2011-03-03)
This paper reports the influence of sequence structures of block copolymers composed of poly(lactic acid-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG) on their thermogelling aqueous behaviors. A series of thermogelling PLGA-PEG-PLGA triblock copolymers with similar chemical compositions and block lengths
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.