905291
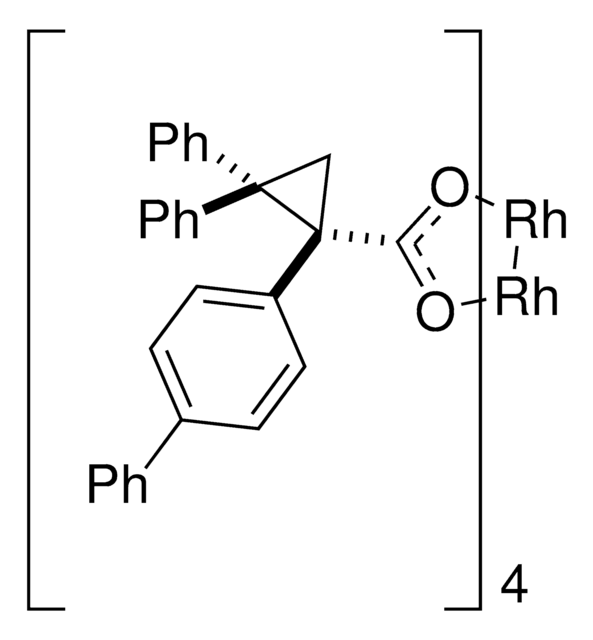
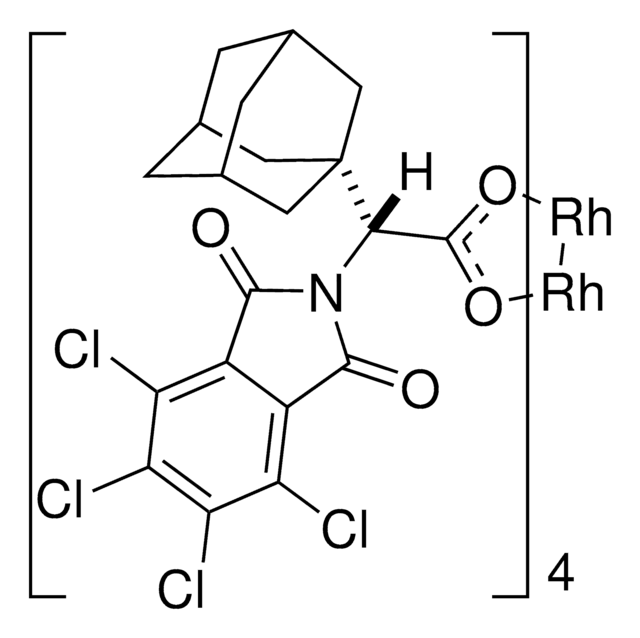
Rh2(R-BTPCP)4
동의어(들):
Davies dirhodium catalyst, Dirhodium tetrakis((S,R)-1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylate), Dirhodium tetrakis[(R)-1-(4-bromophenyl)-2,2- diphenylcyclopropane carboxylate], Tetrakis[(R)-(-)-[(1R)-1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylato]dirhodium(II), Tetrakis[(R)-1-(4-bromophenyl)-2,2- diphenylcyclopropane carboxylato]dirhodium(II)
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C88H64Br4O8Rh2
CAS Number:
Molecular Weight:
1774.87
MDL number:
UNSPSC 코드:
12161600
NACRES:
NA.22
추천 제품
양식
powder or crystals
mp
>300 °C
애플리케이션
Rh catalyst developed by the Davies lab used for enantioselective cyclopropanations and C-H funcionalization under low catalyst loadings.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Daniel Rackl et al.
Organic letters, 19(12), 3055-3058 (2017-06-06)
A tandem reaction system has been developed for the preparation of donor/acceptor-substituted diazo compounds in continuous flow coupled to dirhodium-catalyzed C-H functionalization or cyclopropanation. Hydrazones were oxidized in flow by solid-supported N-iodo-p-toluenesulfonamide potassium salt (PS-SO2NIK) to generate the diazo compounds
Changming Qin et al.
Journal of the American Chemical Society, 133(47), 19198-19204 (2011-11-04)
Dirhodium tetrakis-(R)-(1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylate) (Rh(2)(R-BTPCP)(4)) was found to be an effective chiral catalyst for enantioselective reactions of aryl- and styryldiazoacetates. Highly enantioselective cyclopropanations, tandem cyclopropanation/Cope rearrangements and a combined C-H functionalization/Cope rearrangement were achieved using Rh(2)(R-BTPCP)(4) as catalyst. The advantages of Rh(2)(R-BTPCP)(4)
Ji-Min Yang et al.
Journal of the American Chemical Society, 139(10), 3784-3789 (2017-02-15)
Herein, we report transition-metal-catalyzed B-H bond insertion reactions between borane adducts and alkynes to afford organoboron compounds in excellent yields under mild reaction conditions. This successful use of alkynes as carbene precursors in these reactions constitutes a new route to
Changming Qin et al.
Organic letters, 15(2), 310-313 (2013-01-05)
The rhodium-catalyzed reaction of 2-diazo-5-arylpent-4-enoates can be controlled by the appropriate choice of catalyst and catalyst loading to form either 2-arylbicyclo[1.1.0]butane carboxylates or cyclohexene derivatives. Both products are produced in a highly diastereoselective manner, with 2-arylbicyclo[1.1.0]butane carboxylates preferentially formed under
Kuangbiao Liao et al.
Nature, 551(7682), 609-613 (2017-11-21)
The synthesis of complex organic compounds usually relies on controlling the reactions of the functional groups. In recent years, it has become possible to carry out reactions directly on the C-H bonds, previously considered to be unreactive. One of the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)