745979
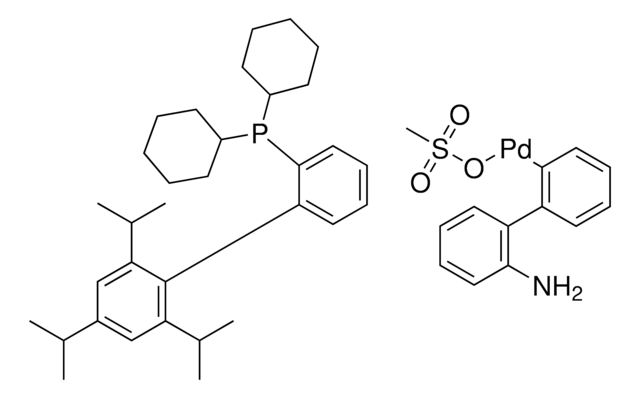
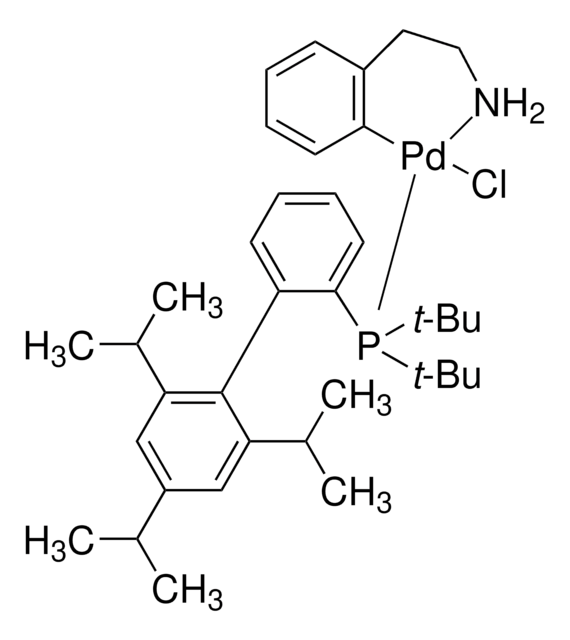
tBuBrettPhos Pd G3
96%
동의어(들):
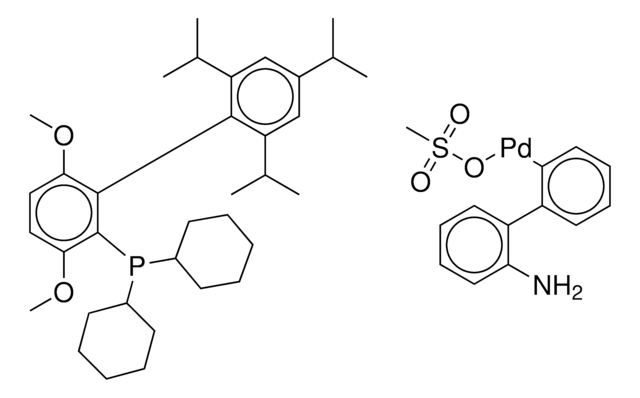
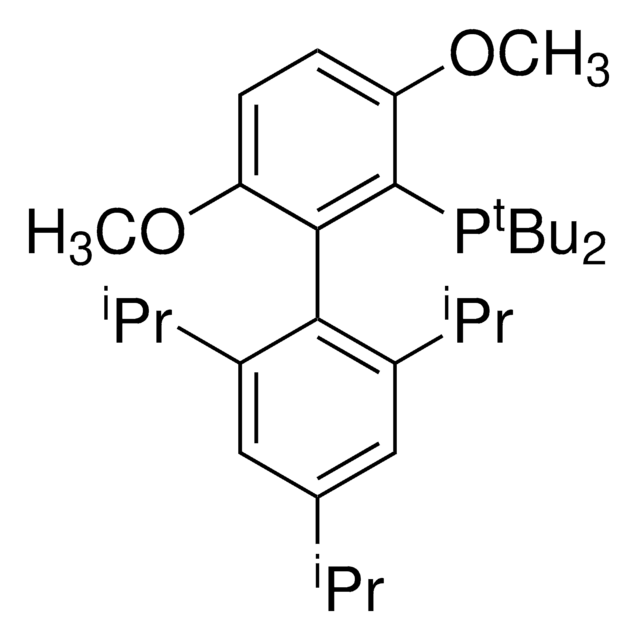
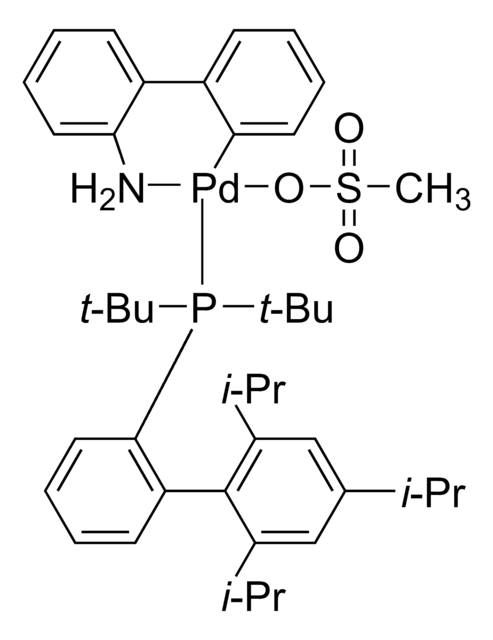
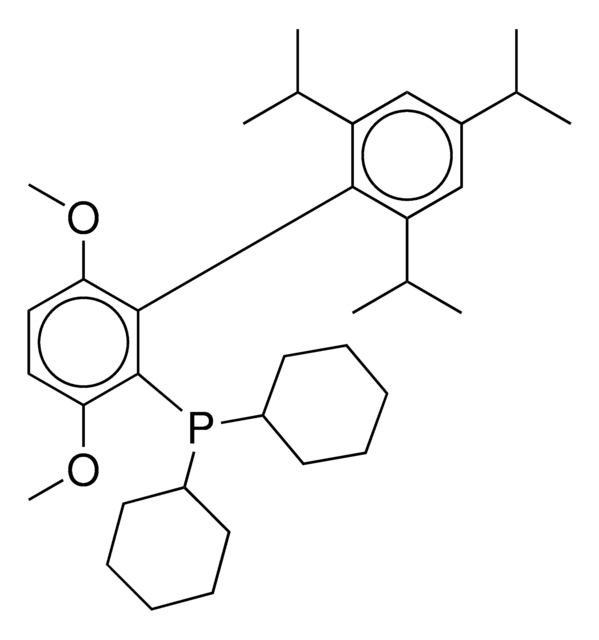
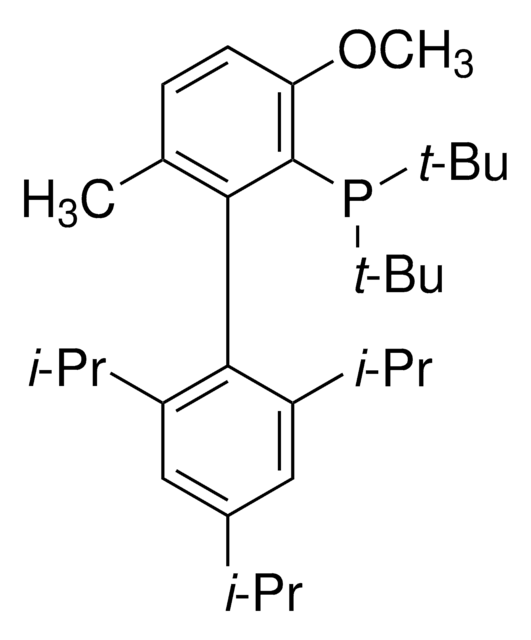
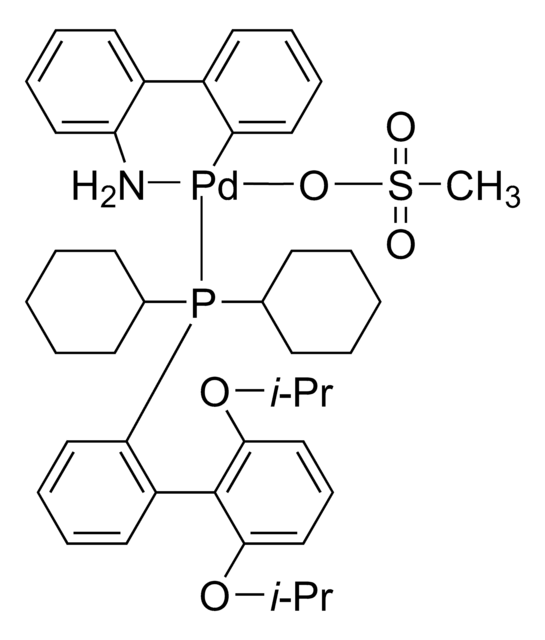
tert-BuBrettPhos-Pd-G3, [(2-Di-tert-butylphosphino-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
추천 제품
Quality Level
분석
96%
형태
solid
특징
generation 3
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
119-131 °C
작용기
phosphine
SMILES string
COC1=CC=C(C(P(C(C)(C)C)C(C)(C)C)=C1C2=C(C=C(C=C2C(C)C)C(C)C)C(C)C)OC.NC3=C(C=CC=C3)C4=C(C=CC=C4)[Pd]OS(C)(=O)=O
InChI
1S/C31H49O2P.C12H10N.CH4O3S.Pd/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h15-21H,1-14H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI key
GAQPAUHHECNHNS-UHFFFAOYSA-M
일반 설명
애플리케이션
For small scale and high throughput uses, product is also available as ChemBeads (928313)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
관련 콘텐츠
Cross-coupling is a common reaction in organic chemistry for the creation of C-C, C-N, and C-O bonds with the aid of a metal catalyst.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.