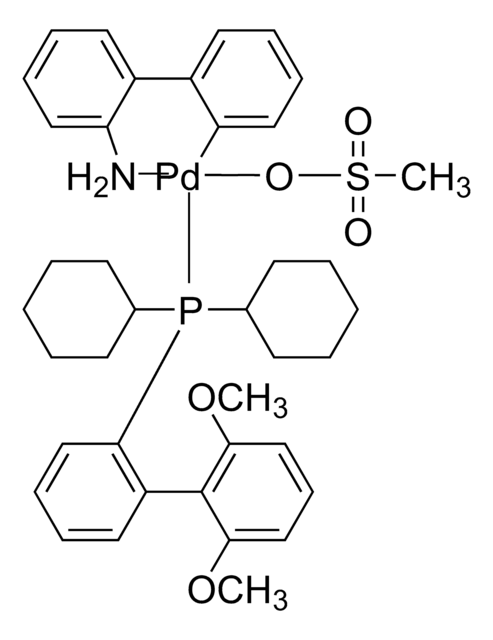
732117
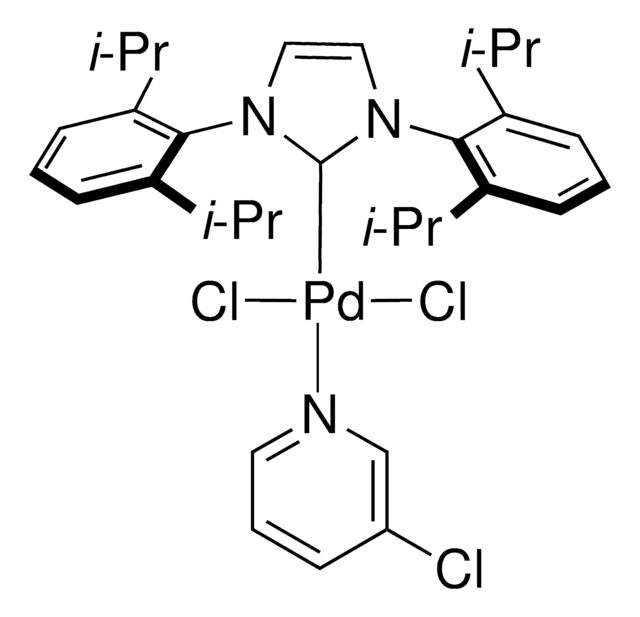
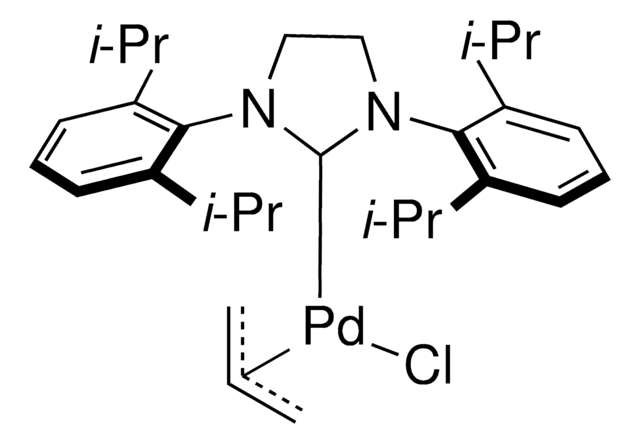
Pd-PEPPSI™-IPent catalyst
≥95%
동의어(들):
Dichloro[1,3-bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II), [1,3-Bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)dichloropalladium(II), [1,3-Bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
추천 제품
Quality Level
분석
≥95%
형태
solid
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
195-201 °C
저장 온도
−20°C
SMILES string
Clc1cccnc1.CCC(CC)c2cccc(C(CC)CC)c2N3C=CN(c4c(cccc4C(CC)CC)C(CC)CC)\C3=[Pd](/Cl)Cl
InChI
1S/C35H52N2.C5H4ClN.2ClH.Pd/c1-9-26(10-2)30-19-17-20-31(27(11-3)12-4)34(30)36-23-24-37(25-36)35-32(28(13-5)14-6)21-18-22-33(35)29(15-7)16-8;6-5-2-1-3-7-4-5;;;/h17-24,26-29H,9-16H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
InChI key
BCXSKTXOKALLAZ-UHFFFAOYSA-L
일반 설명
애플리케이션
Cross-Coupling, Amination and Heck Transformation using PEPPSI Catalysts
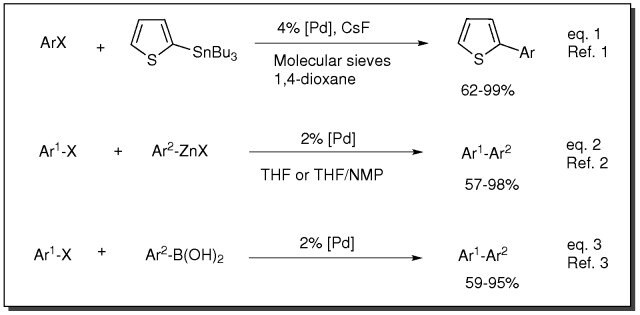
- Catalyst for Stille coupling reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
For small scale and high throughput uses, product is also available as ChemBeads (928399)
법적 정보
관련 제품
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
Professor Mike Organ at York University, along with co-workers Dr. Chris O’Brien and Dr. Eric Kantchev, have developed an palladium N-heterocyclic-carbene (NHC) catalyst system. They reacted PdCl2with a bulky NHC ligand, 2,6-diisopropylphenyllimidazolium chloride (IPr), and an α-donating 3-chloropyridine ligand for stability. The title complex, PEPPSI™, stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation. Sigma-Aldrich offesr gram-scale quantities of the PEPPSI™ catalyst in collaboration with the Organ research group.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Multiple tools have been created to ensure your success with kit set up. Start with the more detailed guide to ensure you are comfortable with all of the steps before using the quick guides on the excel worksheet. Remember that while the technique is new, it is still organic chemistry and so the steps will seem easy once you try just one kit. It is just a new way of approaching something you are already very good at.
관련 콘텐츠
Cross-coupling is a common reaction in organic chemistry for the creation of C-C, C-N, and C-O bonds with the aid of a metal catalyst.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.