723614
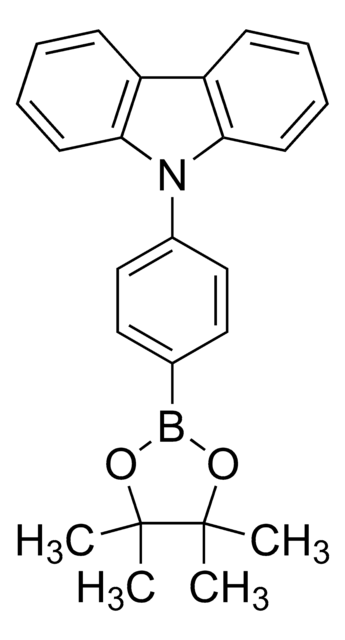
4-(Diphenylamino)phenylboronic acid pinacol ester
95%
동의어(들):
N-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl-N-phenylbenzenamine, Diphenyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-amine
About This Item
추천 제품
분석
95%
양식
powder
mp
93-98 °C
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(cc2)N(c3ccccc3)c4ccccc4
InChI
1S/C24H26BNO2/c1-23(2)24(3,4)28-25(27-23)19-15-17-22(18-16-19)26(20-11-7-5-8-12-20)21-13-9-6-10-14-21/h5-18H,1-4H3
InChI key
VKSWIFGDKIEVFZ-UHFFFAOYSA-N
일반 설명
애플리케이션
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.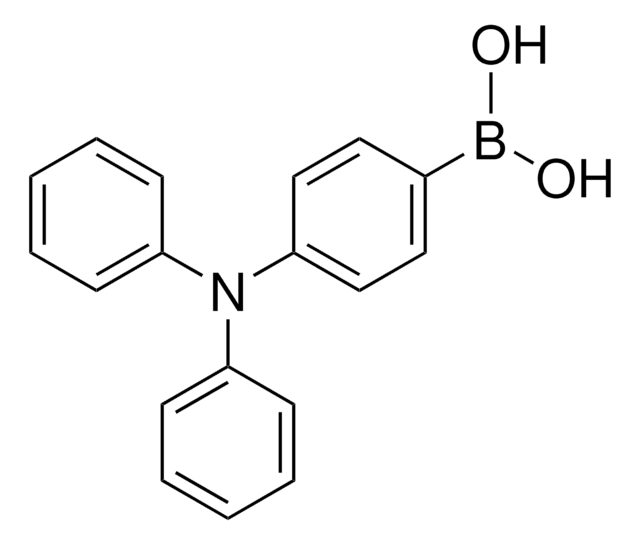
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
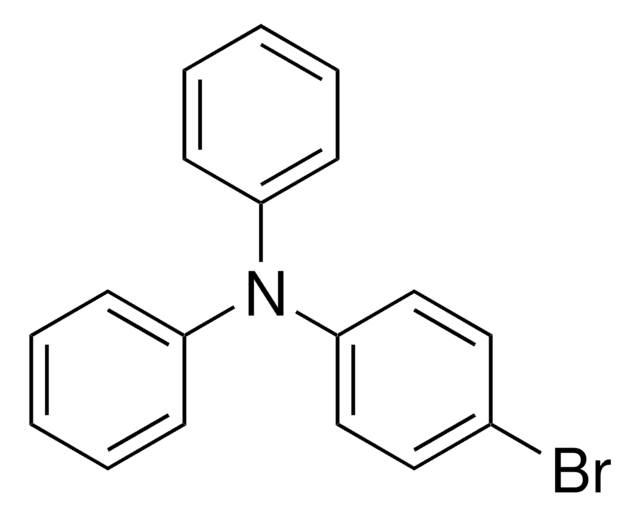
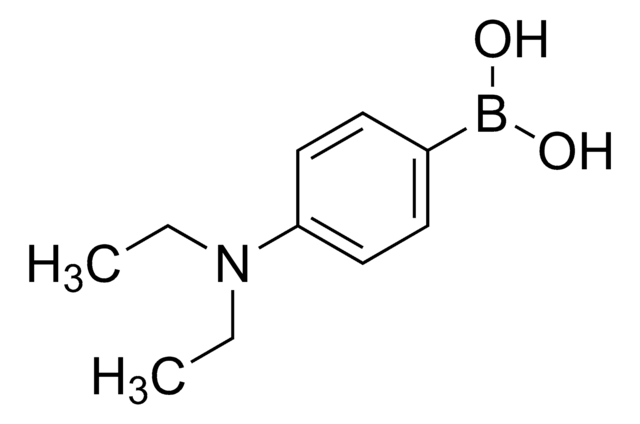

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)

