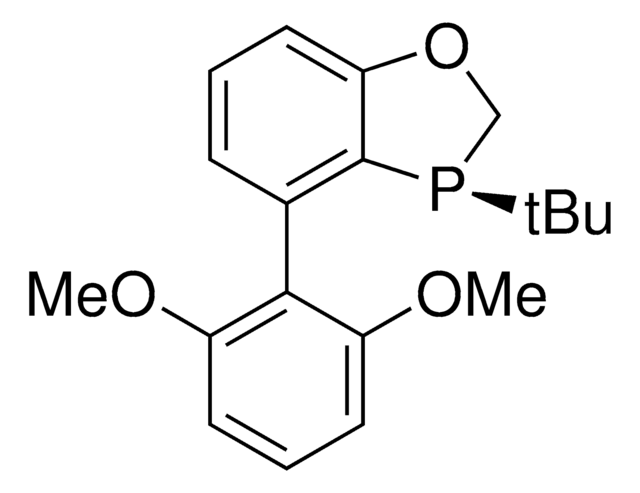
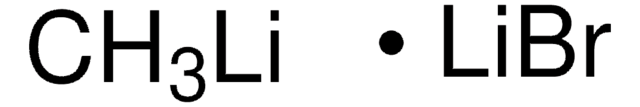About This Item
추천 제품
분석
≥94%
양식
solid
광학 활성
[α]20/D +94°, c = 0.5 in chloroform
SMILES string
COc1ccc2ccccc2c1-c3c(ccc4ccccc34)P(c5ccccc5)c6ccccc6
InChI
1S/C33H25OP/c1-34-30-22-20-24-12-8-10-18-28(24)32(30)33-29-19-11-9-13-25(29)21-23-31(33)35(26-14-4-2-5-15-26)27-16-6-3-7-17-27/h2-23H,1H3
InChI key
KRWTWSSMURUMDE-UHFFFAOYSA-N
일반 설명
애플리케이션
Ligand used in palladium-catalyzed asymmetric hydrosilylation of olefins, palladium-catalyzed reduction of allylic esters, rhodium-catalyzed asymmetric addition reactions, and asymmetric amination reactions catalyzed by copper(I) complexes.
법적 정보
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
문서
In the last few years, a number of chiral diene ligands have emerged for a variety of asymmetric transformations.1 This powerful new method has proven to be an efficient way for the construction of enantioenriched compounds from achiral substrates.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)
![(S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4- a′]dinaphthalen-4-yl)dimethylamine 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)

![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)