모든 사진(1)
About This Item
실험식(Hill 표기법):
C6H8O2S
CAS Number:
Molecular Weight:
144.19
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
분석
97%
refractive index
n20/D 1.5409
bp
100-102 °C/10-11 mmHg
density
1.209 g/mL at 25 °C
저장 온도
−20°C
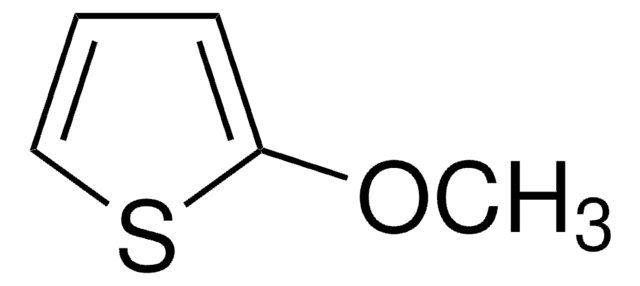
SMILES string
COc1cscc1OC
InChI
1S/C6H8O2S/c1-7-5-3-9-4-6(5)8-2/h3-4H,1-2H3
InChI key
ZUDCKLVMBAXBIF-UHFFFAOYSA-N
일반 설명
3,4-Dimethoxythiophene (DMOT) is a monomer and a precursor which can be synthesized by ring closure reaction of 2,3-dimethoxy-1,3-butadiene and sulfur dichloride in hexane medium. It is an oligothiphene that is majorly used in the development of electroactive materials for organic electronics based applications.
애플리케이션
Building block in the synthesis of an N2S2-N4 porphyrin dyad used to study photoinduced energy transfer.
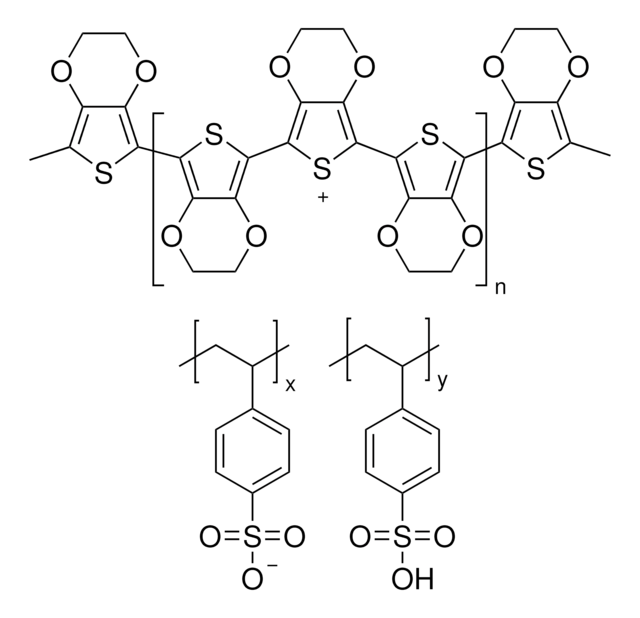
DMOT can be trans-esterified to form 3,4-ethylenendioxythiophene (EDOT). It can further be polymerized to produce PEDOT which can be used as a conductive polymer in π-conjugated systems. It can be polymerized to form poly(dimethoxythiphenes) which can potentially be used in the fabrication energy storage devices on electrochemical doping.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
224.1 °F - closed cup
Flash Point (°C)
106.7 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Revisiting the electropolymerization of 3, 4-dimethoxythiophene in organic and micellar media.
Fall M, et al.
Synthetic Metals, 123(3), 365-372 (2001)
Sokkalingam Punidha et al.
The Journal of organic chemistry, 73(1), 323-326 (2007-12-12)
Click chemistry has been successfully applied in the synthesis of the first example of a triazole-bridged porphyrin dyad containing N(2)S(2) porphyrin and N(4) or ZnN(4) porphyrin subunits, and fluorescence study indicated a possibility of singlet-singlet energy transfer from the N(4)
In situ conductance studies of p-and n-doping of poly (3, 4-dialkoxythiophenes).
Skompska M, et al.
Journal of Electroanalytical Chemistry, 577(1), 9-17 (2005)
Biomimetic Synthesis of Water Soluble Conductive Polypyrrole and Poly (3, 4 ethylenedioxythiophene).
Bruno FF, et al.
MRS Online Proceedings Library, 736(4), 607-609 (2002)
Thieno [3, 4-b]-1, 4-oxathiane: An Unsymmetrical Sulfur Analogue of 3, 4-Ethylenedioxythiophene (EDOT) as a Building Block for Linear pi-Conjugated Systems.
Blanchard P, et al.
Organic Letters, 4(4), 607-609 (2002)
문서
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![2-Chloromethyl-2,3-dihydrothieno[3,4-b]-1,4-dioxine 95%](/deepweb/assets/sigmaaldrich/product/structures/422/187/4cc7b858-9e06-4ce2-8d39-d817b8313964/640/4cc7b858-9e06-4ce2-8d39-d817b8313964.png)

![2,3-Dihydrothieno[3,4-b][1,4]dioxine-5-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/145/904/fa7794c7-33ba-4c32-99c7-e9a63b506736/640/fa7794c7-33ba-4c32-99c7-e9a63b506736.png)