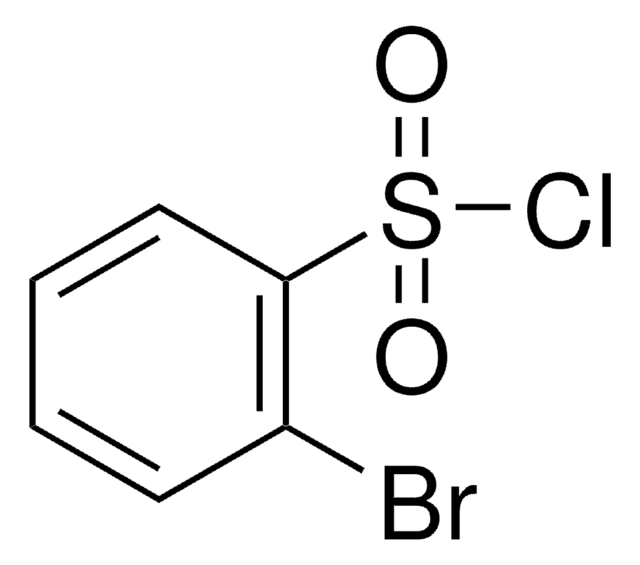
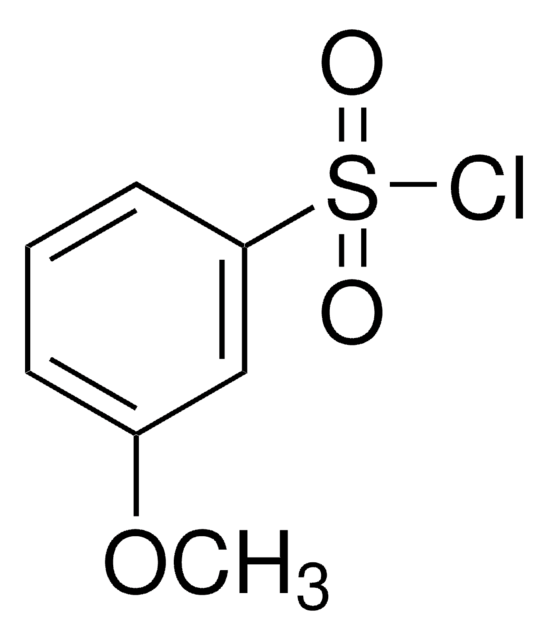
추천 제품
Quality Level
분석
96%
refractive index
n20/D 1.593 (lit.)
bp
90-91 °C/0.5 mmHg (lit.)
mp
30-33 °C
density
1.773 g/mL at 25 °C (lit.)
작용기
bromo
SMILES string
ClS(=O)(=O)c1cccc(Br)c1
InChI
1S/C6H4BrClO2S/c7-5-2-1-3-6(4-5)11(8,9)10/h1-4H
InChI key
PJGOLCXVWIYXRQ-UHFFFAOYSA-N
일반 설명
3-Bromobenzenesulfonyl chloride is an aryl sulfonyl chloride derivative. It participates in the synthesis of N-sulfonylanthranilic acid derivatives and potent P1′ benzenesulfonyl azacyclic urea human immunodeficiency virus (HIV) protease inhibitors.
애플리케이션
3-Bromobenzenesulfonyl chloride may be used in the preparation of:
- 2-(3-bromophenyl)-5-n-butylfuran
- 2-(3-bromophenyl)-3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran
- 3-bromo-4-(3-bromophenyl)thiophene
- 2,5-bis(3-bromophenyl)-1-methylpyrrole
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Pd?Catalysed Direct Arylation of Heteroaromatics Using (Poly) halobenzenesulfonyl Chlorides as Coupling Partners: One Step Access to (Poly) halo?Substituted Bi (hetero) aryls.
Skhiri A, et al.
European Journal of Organic Chemistry, 2015(20), 4428-4436 (2015)
Peggy P Huang et al.
Bioorganic & medicinal chemistry letters, 14(15), 4075-4078 (2004-07-01)
A series of novel azacyclic urea HIV protease inhibitors bearing a benzenesulfonamide group at P1' were synthesized utilizing a parallel synthesis method. Structural studies of early analogs bound in the enzyme active site were used to design more potent inhibitors.
Zheng Yin et al.
Journal of medicinal chemistry, 52(24), 7934-7937 (2009-12-18)
A novel class of compounds containing N-sulfonylanthranilic acid was found to specifically inhibit dengue viral polymerase. The structural requirements for inhibition and a preliminary structure-activity relationship are described. A UV cross-linking experiment was used to map the allosteric binding site
문서
Aryl sulfonyl chloride derivatives are frequently used in parallel synthesis to synthesize sulfonamides and sulfonate linkages.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 545716-1G | 4061837804267 |
| 545716-5G | 4061837804274 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.