모든 사진(3)
About This Item
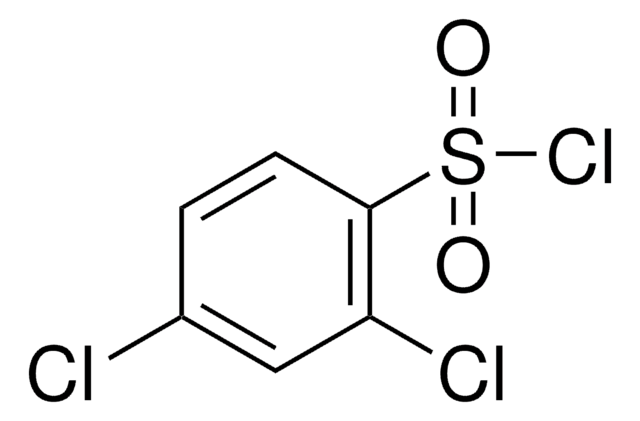
Linear Formula:
Cl2C6H3SO2Cl
CAS Number:
Molecular Weight:
245.51
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
53-56 °C (lit.)
SMILES string
Clc1cccc(Cl)c1S(Cl)(=O)=O
InChI
1S/C6H3Cl3O2S/c7-4-2-1-3-5(8)6(4)12(9,10)11/h1-3H
InChI key
WGGKQIKICKLWGN-UHFFFAOYSA-N
일반 설명
2,6-Dichlorobenzenesulfonyl chloride, also known as 2,6-dichlorophenylsulfonyl chloride, is an aryl sulfonyl chloride derivative.
애플리케이션
2,6-Dichlorobenzenesulfonyl chloride may be used as a starting material in the multi-step synthesis of sulfonamide-containing diarylsquaramide. It may also be used in the synthesis of cycloheptyl substituted 1,2,4-triazolopyridine (TZP) analogs.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Haixia Wang et al.
Bioorganic & medicinal chemistry letters, 21(14), 4146-4149 (2011-06-22)
A series of pyridyl amide/sulfonamide inhibitors of 11β-HSD-1 were modified to incorporate a novel 1,2,4-triazolopyridine scaffold. Optimization of substituents at the 3 and 8 position of the TZP core, with a special focus on enhancing metabolic stability, resulted in the
Brent W McCleland et al.
Bioorganic & medicinal chemistry letters, 17(6), 1713-1717 (2007-01-24)
N,N'-diarylsquaramides were prepared and evaluated as antagonists of CXCR2. The compounds were found to be potent and selective antagonists of CXCR2. Significant differences in SAR was observed relative to the previously described N,N'-diarylurea series. As was the case in the
문서
Aryl sulfonyl chloride derivatives are frequently used in parallel synthesis to synthesize sulfonamides and sulfonate linkages.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.