모든 사진(2)
About This Item
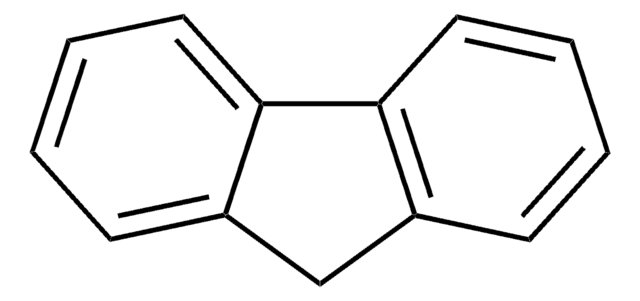
실험식(Hill 표기법):
C21H26
CAS Number:
Molecular Weight:
278.43
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
mp
121-124 °C (lit.)
SMILES string
CC(C)(C)c1ccc-2c(Cc3cc(ccc-23)C(C)(C)C)c1
InChI
1S/C21H26/c1-20(2,3)16-7-9-18-14(12-16)11-15-13-17(21(4,5)6)8-10-19(15)18/h7-10,12-13H,11H2,1-6H3
InChI key
DFZYPLLGAQIQTD-UHFFFAOYSA-N
관련 카테고리
일반 설명
2,7-Di-tert-butylfluorene can be synthesized by reacting fluorene, CS2 and FeCl3 and 2-chloro-2-methylpropane. It can also be obtained by reacting fluorene with tert-butyl chloride in the presence of FeCl3.
애플리케이션
2,7-Di-tert-butylfluorene may be used in the preparation of:
- 2,7-di-tert-butyl-9-fluorenylmethanol
- 2,7-di-tert-butyl-9-[ [(p-chlorophenyl)amino]methylene]-fluorene
- dihydrocyclohepta[def]fluorene
- new group 4 metal complexes containing aminofluorenyl ligands
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
Miller SA and Bercaw JE.
Organometallics, 19, 5608-5608 (2000)
Synthesis and Properties of Kinetically Stabilized Cyclohepta [def] fluorene Derivatives.
Grieser, UD and Hafner K.
Chemische Berichte, 127(11), 2307-2314 (1994)
Investigation of the reaction between amino acids or amino acid esters and 9-formylfluorene and its equivalents. Possible utility of the derived enamines as amino group protectants.
Carpino LA, et al.
The Journal of Organic Chemistry, 54(18), 4302-4313 (1989)
Fmoc: a more soluble analogue of the 9-fluorenylmethoxycarbonyl protecting group.
K D Stigers et al.
The Journal of organic chemistry, 65(12), 3858-3860 (2000-06-24)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.