추천 제품
분석
≥98%
mp
104-106 °C (lit.)
solubility
acetone: 25 mg/mL, clear, colorless
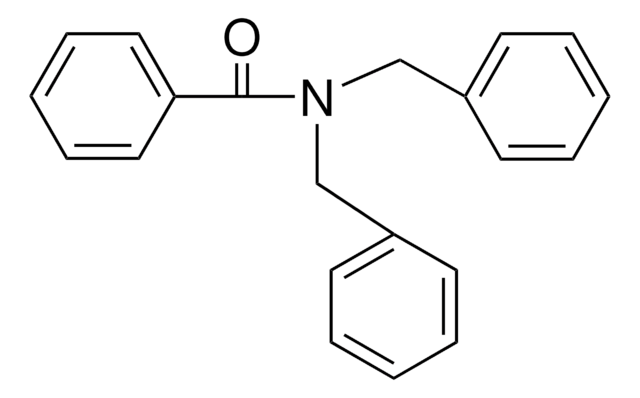
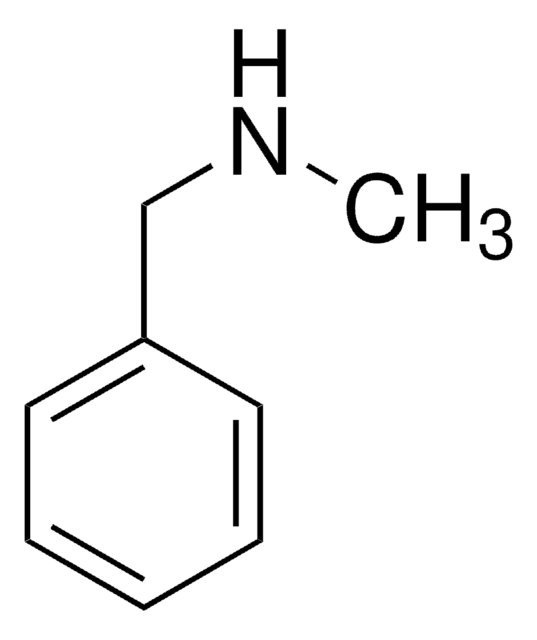
SMILES string
O=C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H13NO/c16-14(13-9-5-2-6-10-13)15-11-12-7-3-1-4-8-12/h1-10H,11H2,(H,15,16)
InChI key
LKQUCICFTHBFAL-UHFFFAOYSA-N
일반 설명
N-Benzylbenzamide inhibits the activity of tyrosinase.
애플리케이션
A convenient precursor to α-substituted benzylamines and an indicator for the titration of butyllithium and other lithium bases. For references see Aldrichimica Acta .
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Aldrichimica Acta, 11, 20-20 (1978)
Yae Eun Chong et al.
Biomedical chromatography : BMC, 33(11), e4653-e4653 (2019-07-20)
Ondansetron, a widely used antiemetic agent, is a P-glycoprotein (P-gp) substrate and therefore expression of P-gp at the blood-brain barrier limits its distribution to the central nervous system (CNS), which was observed to be reversed by coadministration with P-gp inhibitors.
Zsanett Dorkó et al.
Talanta, 162, 167-173 (2016-11-14)
A simple and efficient method is presented for assessing molecularly imprinted polymers (MIP) and other sorbents from the point of view of practical applications. The adsorption isotherms of the compounds, which need to be separated or detected in an application
Sung Jin Cho et al.
Bioorganic & medicinal chemistry letters, 16(10), 2682-2684 (2006-03-04)
A series of potent inhibitors of tyrosinase and their structure-activity relationships are described. N-Benzylbenzamide derivatives (1-21) with hydroxyl(s) were synthesized and tested for their tyrosinase inhibitory activity. With this series, compound 15 provided a potent tyrosinase inhibition: it effectively inhibited
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.