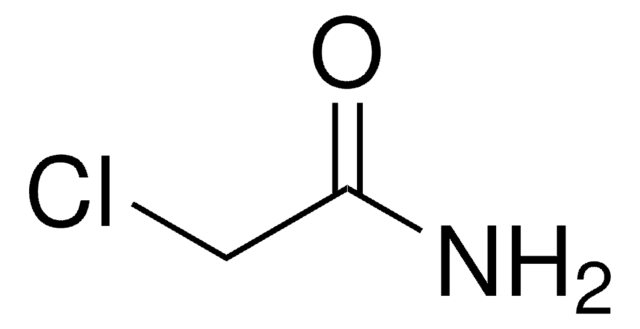
추천 제품
일반 설명
Cyanogen bromide (CNBr) is a halogen halide. Its utility as a condensing agent during chemical ligation of oligodeoxyribonucleotides has been reported. Its threshold photoelectron (TPE) spectrum has been recorded under high resolution. The cryopolymerization of CNBr on irradiation has been investigated.
애플리케이션
Cyanogen bromide may be used:
- To activate polysaccharides for coupling with erythrocytes.
- As an oxidizing agent in the urine thaimine assay.
- In the preparation of 2-amino-5-(2′-thienyl)-1,3,4-oxadiazole.
Reaction with C60Ph5Cl produces a novel phenylated isoquinolino[3′,4′:1,2][60]fullerene.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1B
보충제 위험성
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
>149.0 °F - closed cup
Flash Point (°C)
> 65 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges, type P3 (EN 143) respirator cartridges
N I Sokolova et al.
FEBS letters, 232(1), 153-155 (1988-05-09)
Cyanogen bromide was found to condense oligodeoxyribonucleotides on a complementary template in aqueous solution. Optimum conditions for this vigorous and effective reaction were developed. CNBr proved to be useful for incorporation of phosphoramidate or pyrophosphate internucleotide bonds in DNA duplexes.
Threshold photoelectron spectroscopy of cyanogen bromide.
Yencha AJ, et al.
Chemical Physics Letters, 392(1), 202-208 (2004)
Oxadiazole: a biologically important heterocycle.
Somani RR and Shirodkar PY.
Der Pharma Chemica, 1(1), 130-140 (2009)
Cyanogen bromide, a good reagent for assay of thiamine in urine.
K Ratanaubolchai et al.
Clinical chemistry, 25(9), 1670-1671 (1979-09-01)
Low-temperature radiation polymerization of cyanogen bromide.
Kichigina GA, et al.
Mendeleev Communications, 8(4), 159-160 (1998)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.