추천 제품
Quality Level
분석
97%
mp
153-157 °C (lit.)
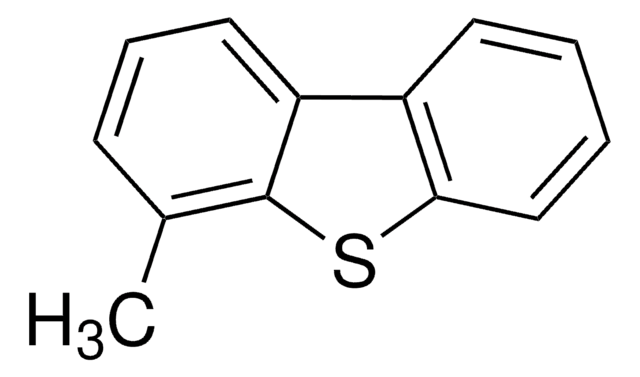
SMILES string
Cc1cccc2c3cccc(C)c3sc12
InChI
1S/C14H12S/c1-9-5-3-7-11-12-8-4-6-10(2)14(12)15-13(9)11/h3-8H,1-2H3
InChI key
MYAQZIAVOLKEGW-UHFFFAOYSA-N
일반 설명
4,6-Dimethyldibenzothiophene (4,6-DMDBT), a high refractory sulfur compound, is commonly found in diesel fuel. Its synthesis has been reported. The hydrodesulfurization of 4,6-DMDBT using bulk nickel alloy, bulk tungsten phosphide (WP), NiMo sulfide supported on active carbons and molecularly imprinted polymers have been reported. The dielectric behavior of 4,6-DMDBT under different microwave frequencies and temperature has been investigated.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Meiqin Zheng et al.
Journal of hazardous materials, 362, 424-435 (2018-09-28)
In this work, the adsorption desulfurization performance and adsorption diffusion study of B2O3 modified Ag-CeOx/TiO2-SiO2 adsorbent were investigated. The adsorption desulfurization performance was studied by batch and fixed bed tests. The homogeneous surface diffusion model (HSDM) was employed to investigate
Hussein N Nassar et al.
Environmental science and pollution research international, 28(7), 8102-8116 (2020-10-14)
One of the main precursors of air pollution and acid rains is the presence of the recalcitrant thiophenic compounds, for example dibenzothiophene (DBT) and its derivatives in transportation fuels. In an attempt to achieve the worldwide regulations of ultra-low sulfur
Hydrodesulfurization kinetics and mechanism of 4,6-dimethyldibenzothiophene over NiMo catalyst supported on carbon.
Sakanishi K, et al.
J. Mol. Catal. A: Chem., 155(1), 101-109 (2000)
Development of a novel molecularly imprinted polymer for the retention of 4,6-dimethyldibenzothiophene.
Tom LA, et al.
Microchimica Acta, 176(3-4), 375-380 (2012)
Hydrodesulfurization of dibenzothiophene, 4,6-dimethyldibenzothiophene, and their hydrogenated intermediates over bulk tungsten phosphide.
Yang L, et al.
J. Catal., 330, 330-343 (2015)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 479411-250MG | 4061832373317 |
| 479411-1G | 4061835555215 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.


![11H-Benzo[a]carbazole](/deepweb/assets/sigmaaldrich/product/structures/391/065/abfb4cba-81ab-44b8-a816-d8791a903400/640/abfb4cba-81ab-44b8-a816-d8791a903400.png)