457698
(R)-(+)-2-Methyl-CBS-oxazaborolidine solution
1 M in toluene
동의어(들):
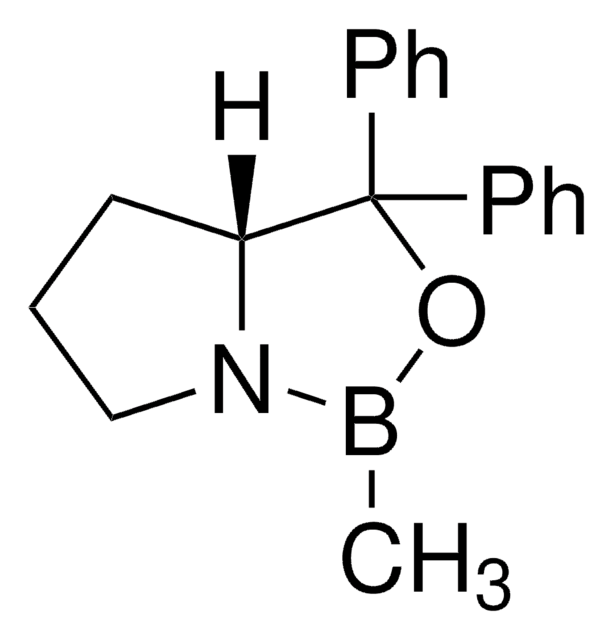
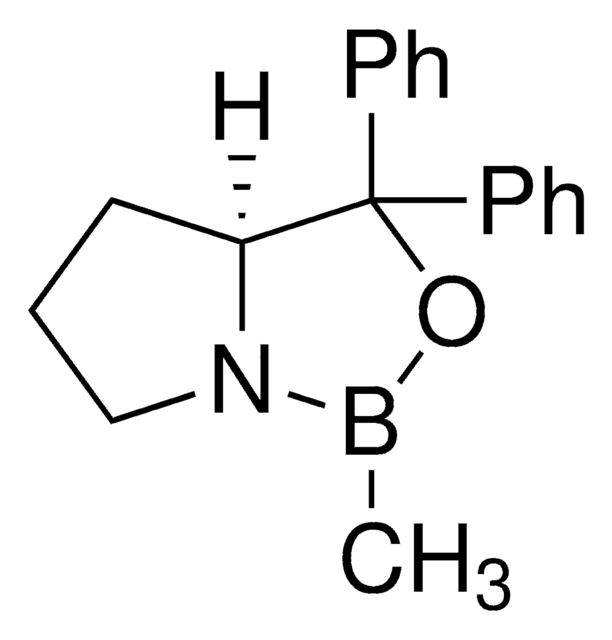
α,α-Diphenyl-D-prolinolmethylboronic acid cyclamide ester, (R)-1-Methyl,3,3-diphenyl-tetrahydro-pyrrolo(1,2-c)(1,3,2)oxazaborole, (R)-Tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborole
About This Item
추천 제품
Quality Level
농도
1 M in toluene
bp
111 °C
density
0.95 g/mL at 25 °C
작용기
phenyl
저장 온도
room temp
SMILES string
[H][C@]12CCCN1B(C)OC2(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m1/s1
InChI key
VMKAFJQFKBASMU-QGZVFWFLSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
It may also be used in the preparation of:
- (-)-diospongin B
- (1R)-2-azido-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanol
- (S)-α-deuteriobenzyl alcohol
- (3S,4R,5S)-1-(trimethylsilyl)-4,5-epoxyhex-1-yn-3-ol
Used in a desymmetrizing reduction leading to (S)-4-hydroxycyclohexenone.
물리적 형태
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
39.2 °F - closed cup
Flash Point (°C)
4 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
문서
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
관련 콘텐츠
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.