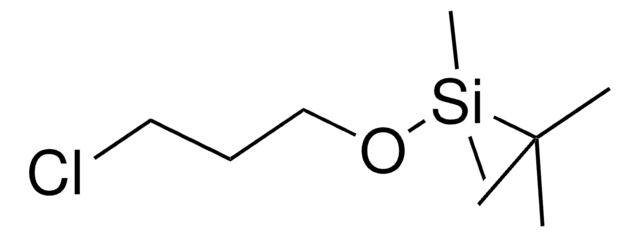
429066
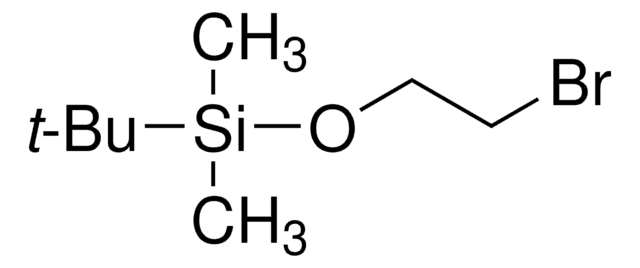
(3-Bromopropoxy)-tert-butyldimethylsilane
97%
동의어(들):
(3-Bromopropoxy)(1,1-dimethylethyl)dimethylsilane, 1-((tert-Butyldimethylsilyl)oxy)-3-bromopropane, 1-Bromo-3-(tert-butyldimethylsiloxy)propane, 1-Bromo-3-[(tert-butyldimethylsilanyl)oxy]propane, 1-Bromo-3-[(tert-butyldimethylsilyl)oxy]propane, 3-((tert-Butyldimethylsilyl)oxy)-1-bromopropane
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
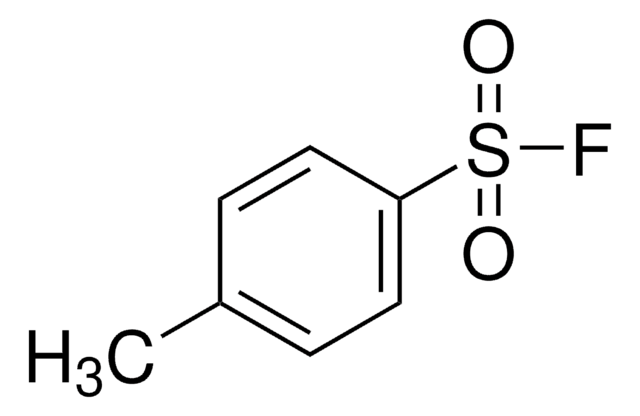
Br(CH2)3OSi(CH3)2C(CH3)3
CAS Number:
Molecular Weight:
253.25
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
liquid
포함
sodium carbonate as stabilizer
refractive index
n20/D 1.451 (lit.)
bp
182 °C (lit.)
density
1.093 g/mL at 25 °C (lit.)
작용기
bromo
저장 온도
2-8°C
SMILES string
CC(C)(C)[Si](C)(C)OCCCBr
InChI
1S/C9H21BrOSi/c1-9(2,3)12(4,5)11-8-6-7-10/h6-8H2,1-5H3
InChI key
QGMROEZDWJTIDW-UHFFFAOYSA-N
일반 설명
(3-Bromopropoxy)-tert-butyldimethylsilane is a bromo silyl ether.
애플리케이션
(3-Bromopropoxy)-tert-butyldimethylsilane may be used to introduce propanol functionality to many pharmaceuticals.
It may be used as an alkylating agent in the synthesis of the following:
It may be used as an alkylating agent in the synthesis of the following:
- N-[2-[N-[3-(tert-butyldimethylsilyloxy)propyl]-N-ethylamino]ethyl]phthalimide
- O-(3-tert-butyldimethylsilyloxypropyl)-N-(tert-butoxycarbonyl)-L-tyrosine methyl ester
- tert-butyldimethyl-[3-(3-methyl-2-nitrophenoxy)propoxy]silane
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
185.0 °F - closed cup
Flash Point (°C)
85 °C - closed cup
이미 열람한 고객
Synthesis of O-(3-[18F] Fluoropropyl)-L-tyrosine (L-[18F] FPT) and its biological evaluation in 9L tumor bearing rat.
Moon BS, et al.
Bull. Korean Chem. Soc., 26(1), 91-96 (2005)
Ramani R Ranatunge et al.
Journal of medicinal chemistry, 47(9), 2180-2193 (2004-04-16)
The synthesis of a series of novel pyrazoles containing a nitrate (ONO(2)) moiety as a nitric oxide (NO)-donor functionality is reported. Their COX-1 and COX-2 inhibitory activities in human whole blood are profiled. Our data demonstrate that pyrazole ring substituents
A concise total synthesis of (+/-)-vigulariol.
J Stephen Clark et al.
Angewandte Chemie (International ed. in English), 46(3), 437-440 (2006-12-06)
Han-Cheng Zhang et al.
Bioorganic & medicinal chemistry letters, 14(12), 3245-3250 (2004-05-20)
A novel series of acyclic 3-(7-azaindolyl)-4-(aryl/heteroaryl)maleimides was synthesized and evaluated for activity against GSK-3beta and selectivity versus PKC-betaII, as well as a broad panel of protein kinases. Compounds 14 and 17c potently inhibited GSK-3beta (IC(50)=7 and 26 nM, respectively) and
Synthesis, radioiodination and in vivo screening of novel potent iodinated and fluorinated radiotracers as melanoma imaging and therapeutic probes.
Maisonial A, et al.
European Journal of Organic Chemistry, 63, 840-853 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.