모든 사진(2)
About This Item
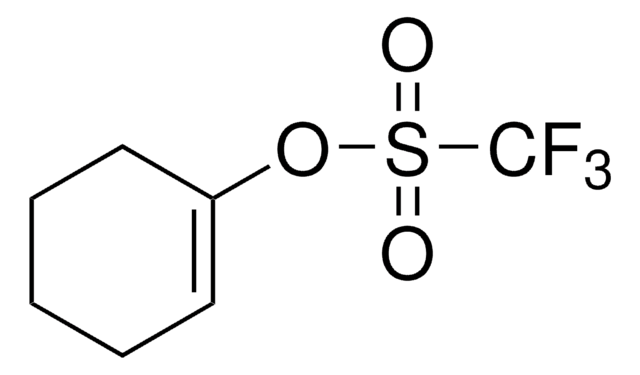
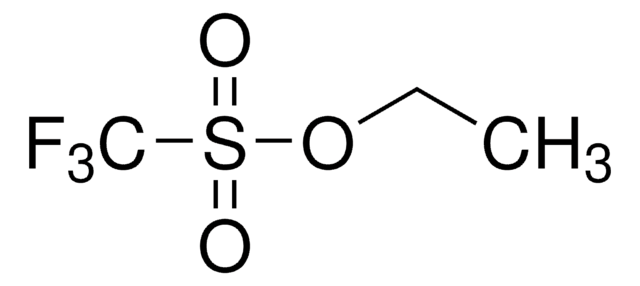
Linear Formula:
C6H5SO3CF3
CAS Number:
Molecular Weight:
226.17
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
양식
liquid
refractive index
n20/D 1.435 (lit.)
bp
99-100 °C/60 mmHg (lit.)
density
1.396 g/mL at 25 °C (lit.)
작용기
fluoro
triflate
SMILES string
FC(F)(F)S(=O)(=O)Oc1ccccc1
InChI
1S/C7H5F3O3S/c8-7(9,10)14(11,12)13-6-4-2-1-3-5-6/h1-5H
InChI key
GRJHONXDTNBDTC-UHFFFAOYSA-N
일반 설명
Phenyl trifluoromethanesulfonate (phenyl triflate) is an aryl fluorosulphonate. It has been synthesized by the reaction of phenol with fluorosulphonic anhydride.
애플리케이션
Phenyl trifluoromethanesulfonate (phenyl triflate) may be used in the following studies:
- As an arylating agent for the asymmetric α-arylation of ketones catalyzed by Pd(dba)2 and difluorphos.
- As a reactant in the one pot synthesis of carbazoles by palladium-catalyzed N-arylation of anilines with phenyl triflate followed by oxidative coupling.
- Synthesis of N-(2,6-diarylbenzoyl)anilines by diarylation of benzanilides with phenyl triflate in the presence of palladium-based catalyst.
- As an arylating agent in the synthesis of (R)-2-phenyl-2,3-dihydrofuran by the arylation of 2,3-dihydrofuran.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
159.8 °F - closed cup
Flash Point (°C)
71 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
One-pot synthesis of carbazoles by palladium-catalyzed N-arylation and oxidative coupling.
Watanabe T, et al.
Chemical Communications (Cambridge, England), 43, 4516-4518 (2007)
Palladium-catalyzed asymmetric arylation of 2, 3-dihydrofuran with phenyl triflate. A novel asymmetric catalysis involving a kinetic resolution process.
Ozawa F, et al.
Organometallics, 12(10), 4188-4196 (1993)
Palladium cross-coupling reactions of aryl fluorosulfonates: an alternative to triflate chemistry.
Roth GP and Fuller CE.
The Journal of Organic Chemistry, 56(11), 3493-3496 (1991)
Xuebin Liao et al.
Journal of the American Chemical Society, 130(1), 195-200 (2007-12-14)
The asymmetric alpha-arylation of ketones with aryl triflates is described, and the use of this electrophile with nickel and palladium catalysts containing a segphos derivative increases substantially the scope of highly enantioselective arylations of ketone enolates. The combination of aryl
Regioselective arylation of benzanilides with aryl triflates or bromides under palladium catalysis.
Kametani Y, et al.
Tetrahedron Letters, 41(15), 2655-2658 (2000)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 423939-5ML | 4061837247682 |
| 423939-25ML | 4061837247675 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.