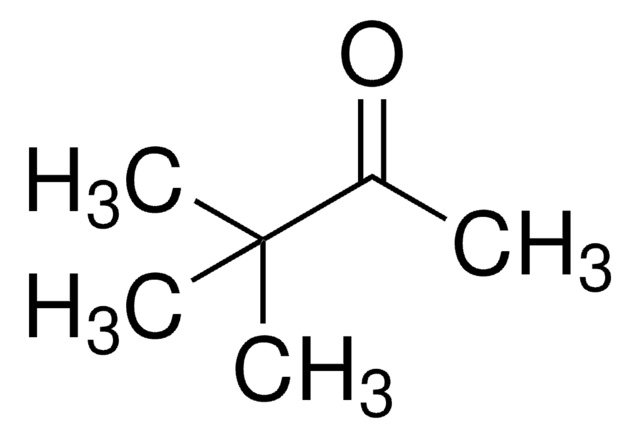
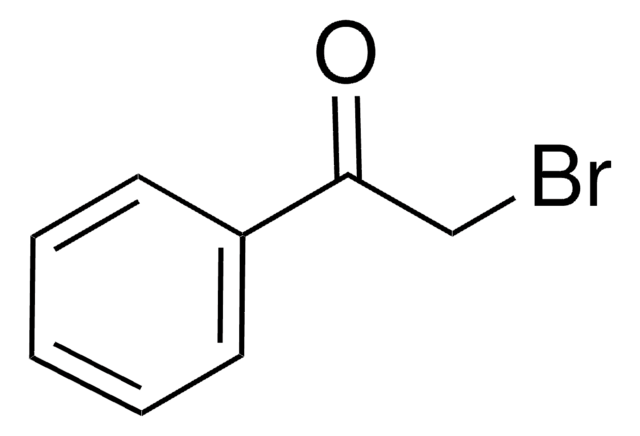
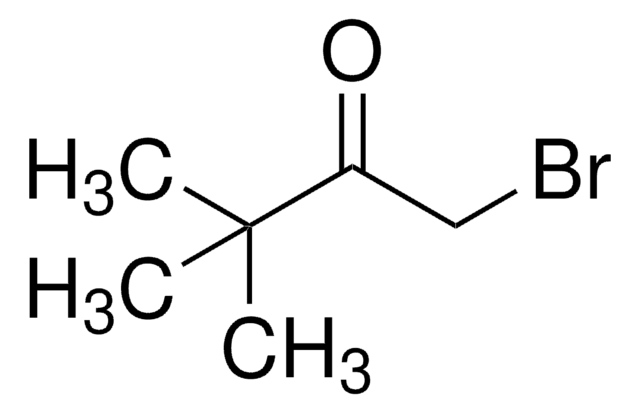
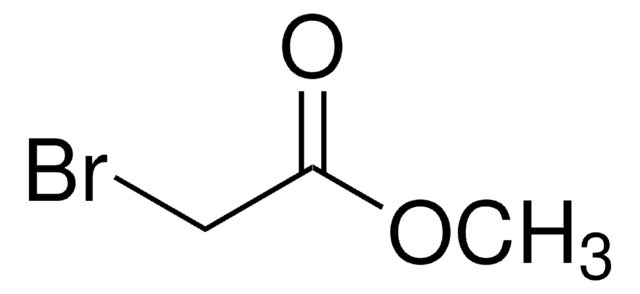
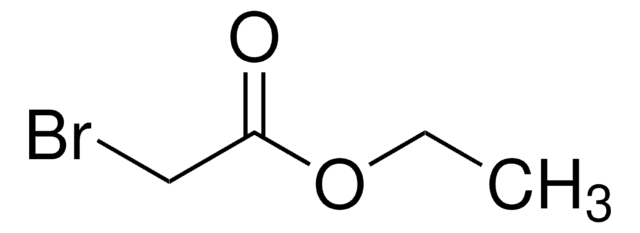
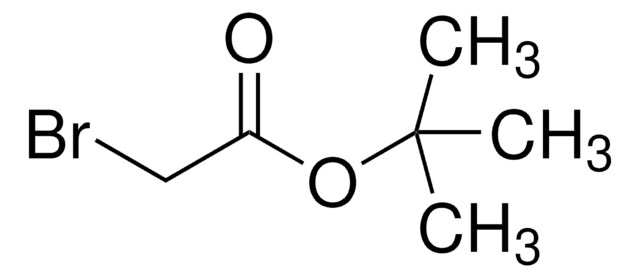
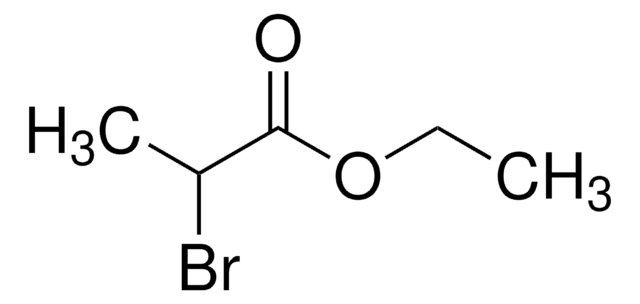
추천 제품
일반 설명
1-Bromopinacolone is an α-bromoketone-aldehyde. Coupling of 1-bromopinacolone with various aldehyde electrophiles catalyzed by SmI2 has been reported. It acts as reversible competitive inhibitor for acetylcholinesterase, during hydrolysis of acetylcholine.
애플리케이션
1-Bromopinacolone is the suitable reagent for the synthesis of a photolabile azido derivative of the kaurene oxidase inhibitor 1-(4- chlorophenyl)4,4-dimethyl-2-(1,2,4-triazol-1-yl) pentan-3-ol (paclobutrazol). It may be used as reagent in the synthesis of 2-t-butyl-6-benzoylimidazo[1,2-b]pyridazine.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
167.0 °F - closed cup
Flash Point (°C)
75 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Brian A Sparling et al.
Organic letters, 10(6), 1291-1294 (2008-02-28)
Highly substituted, very hindered enones were synthesized using a two-step procedure that utilizes a diiodosamarium-promoted Reformatsky-type coupling and dehydration using Martin sulfurane. Both alpha-chloro- and alpha-bromoketones were coupled with a variety of carbonyl nucleophiles to form the intermediate beta-hydroxyketones, occurring
Methyl imidazo [1,2-b] pyridazine-2-carbamates and related compounds as potential antifilarial agents
Mourad AE, et al.
Journal of Heterocyclic Chemistry, 29, 1583-1583 (1992)
D L Hallahan et al.
Plant physiology, 88(4), 1425-1429 (1988-12-01)
A photolabile azido derivative of the kaurene oxidase inhibitor 1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-l-yl) pentan-3-ol (paclobutrazol) has been synthesized for use as a photoaffinity labeling agent. The compound was tested as an inhibitor of the oxidation of ent-kaurene catalyzed by cell-free preparations from endosperm
S G Cohen et al.
The Journal of biological chemistry, 257(23), 14087-14092 (1982-12-10)
1-Bromopinacolone, BrPin, acts initially as a reversible competitive inhibitor for acetylcholinesterase, KI = 0.18 mM in hydrolysis of acetylcholine. Unlike bromoacetone, with time it acts as an irreversible covalent inhibitor. BrPin has a hydrolytic half-life of 30 h at the
S G Cohen et al.
Biochimica et biophysica acta, 997(3), 167-175 (1989-08-31)
1-Bromo-2-[14C]pinacolone, (CH3)3C14COCH2Br [( 14C]BrPin), was prepared from [1-14C]acetyl chloride and tert-butylmagnesium chloride with cuprous chloride catalyst, followed by bromination. It was examined as an active-site directed label for acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) (AcChE). AcChE, isolated from Torpedo nobiliana, has
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.