모든 사진(1)
About This Item
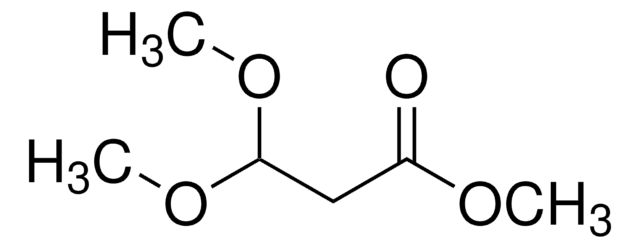
Linear Formula:
(CH3O)2CH(CH2)3CO2CH3
CAS Number:
Molecular Weight:
176.21
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
96%
형태
liquid
refractive index
n20/D 1.422 (lit.)
bp
70-72 °C/2 mmHg (lit.)
density
1.012 g/mL at 25 °C (lit.)
작용기
acetal
ester
ether
SMILES string
COC(CCCC(=O)OC)OC
InChI
1S/C8H16O4/c1-10-7(9)5-4-6-8(11-2)12-3/h8H,4-6H2,1-3H3
InChI key
YOFAONQHOIRLCQ-UHFFFAOYSA-N
일반 설명
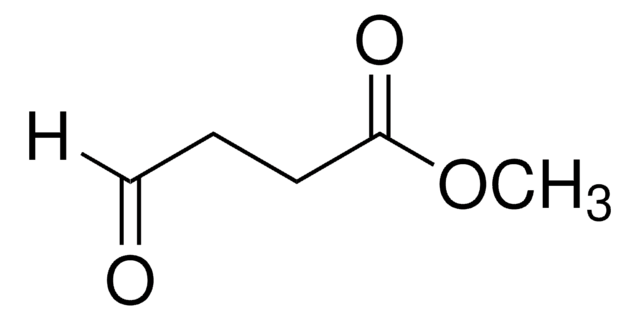
Methyl 5,5-dimethoxyvalerate (methyl 5,5-dimethoxypentanoate) is an ester. It can be prepared by reacting methyl 5-oxopentanoate with p-toluene sulfonic acid and trimethylorthoformate. It participates in the synthesis of 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine.
애플리케이션
Methyl 5,5-dimethoxyvalerate may be employed in the synthesis of seven-membered carbocycles. It may be used in the synthesis of 5-(phenylamino)-4-(phenylimino)methyl)-4-pentenoic acid derivatives.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
145.4 °F - closed cup
Flash Point (°C)
63 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Sanjay Srivastava et al.
The Journal of biological chemistry, 279(51), 53395-53406 (2004-10-07)
Oxidation of unsaturated phospholipids results in the generation of aldehyde side chains that remain esterified to the phospholipid backbone. Such "core" aldehydes elicit immune responses and promote inflammation. However, the biochemical mechanisms by which phospholipid aldehydes are metabolized or detoxified
Fangwei Shao et al.
Bioconjugate chemistry, 19(12), 2487-2491 (2008-12-05)
A facile synthetic route to prepare monofunctional carbocyanine dyes for biological application is developed. Three pentamethine carbocyanine dyes have been successfully modified with a variety of functional groups such as: carboxylic acids, azides, or alkynes. The new dyes are characterized
Formation of seven-membered carbocycles by the use of cyclopropyl silyl ethers as homoenols.
Oleg L Epstein et al.
Angewandte Chemie (International ed. in English), 45(30), 4988-4991 (2006-07-05)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.