추천 제품
Quality Level
분석
99%
양식
solid
mp
49-53 °C (lit.)
작용기
ether
ketone
SMILES string
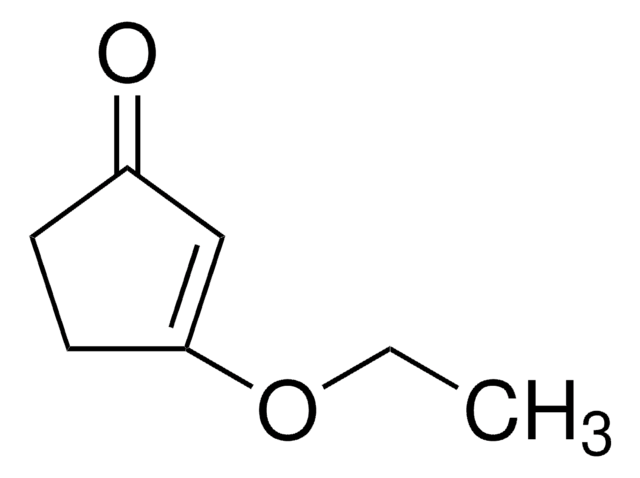
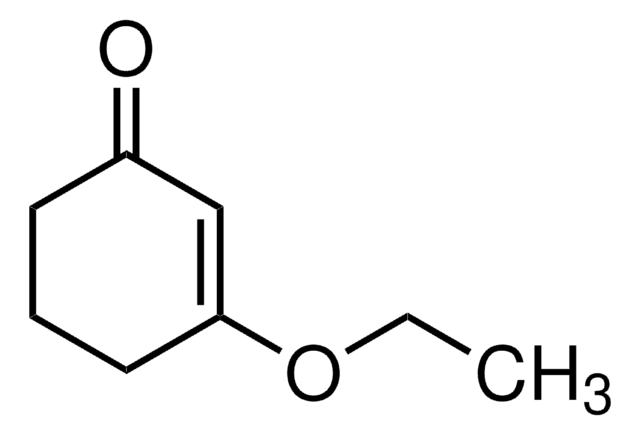
COC1=CC(=O)CC1
InChI
1S/C6H8O2/c1-8-6-3-2-5(7)4-6/h4H,2-3H2,1H3
InChI key
DTWCFCILAJVNPE-UHFFFAOYSA-N
일반 설명
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) is a 3-methoxycycloalk-2- enone.
애플리케이션
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) may be used in the following studies:
- Synthesis of 3-cyclopentyl-2-cyclopenten-1-one.
- As a starting material in the synthesis of 3-alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives and kjellmanianone, an antibiotic.
- As one of the reagent in the synthesis of 3-aryl enones.
- Preparation of 1:1 diastereomeric mixture of TBS (tert-butyldimethylsilyl)-protected enones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Patrick Y Toullec et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(16), 5810-5814 (2004-04-09)
The enantioselective formation of a quaternary stereogenic center coinciding with a hydroxylation process is a very rare reaction from a homogeneous catalysis point of view. Indeed, to our knowledge, no asymmetric transition-metal-catalyzed direct hydroxylation has been reported before. We describe
Tetrahedron, 47, 173-173 (1991)
Discovery of orally efficacious melanin-concentrating hormone receptor-1 antagonists as antiobesity agents. Synthesis, SAR, and biological evaluation of bicyclo [3.1. 0] hexyl ureas.
McBriar MD, et al.
Journal of Medicinal Chemistry, 49(7), 1202-1207 (2012)
Yeonjoon Kim et al.
Bioorganic & medicinal chemistry letters, 24(13), 2807-2810 (2014-05-24)
3-Alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives (1) were studied as a novel class of inhibitors of tumor necrosis factor α (TNF-α) with regard to synthesis and in vitro SAR inhibition of TNF-α. The in vitro IC50 values of these compounds in rat and
Gamal A I Moustafa et al.
The Journal of organic chemistry, 77(2), 1202-1207 (2012-01-03)
The alkylation of dienolates generated from 3-methoxycycloalk-2-enones having a 3'-hydroxyl alkenyl chain provides the corresponding quaternized cycloalkenones in a highly diastereoselective manner. The high degree of stereocontrol in the α-quaternization possibly implies intervention of a rigid chelating transition state that
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.