모든 사진(1)
About This Item
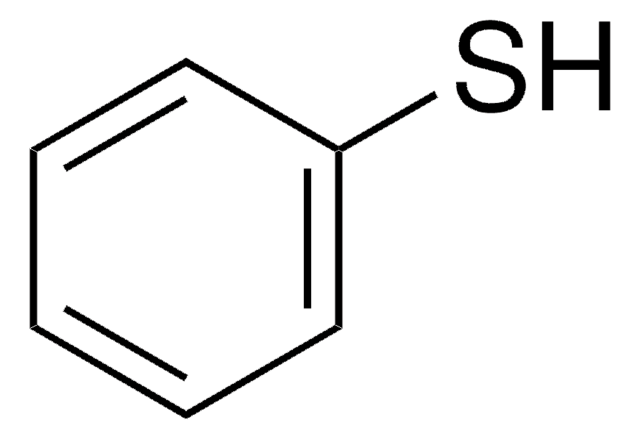
Linear Formula:
C6H5SeH
CAS Number:
Molecular Weight:
157.07
Beilstein:
385715
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Benzeneselenol is an aryl selenol. Diphenyl diselenide-promoted radical addition of benzeneselenol to inactivated acetylenes upon irradiation through Pyrex with a tungsten lamp has been reported. Catalysis of stannane-mediated radical chain reactions by benzeneselenol, generated in situ by reduction of diphenyl diselenide with tributyltin hydride, has been reported.
애플리케이션
Benzeneselenol may be used as starting reagent in the synthesis of monoseleno-substituted 1,3-dienes. It may be used in the synthesis of cationic chalcogenolato-bridged diruthenium complexes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Diphenyl diselenide-promoted radical addition of benzeneselenol to acetylenes.
Ogawa A, et al.
Tetrahedron Letters, 33(10), 1329-1332 (1992)
David Crich et al.
Accounts of chemical research, 40(6), 453-463 (2007-05-11)
The discovery and development of the catalysis of stannane-mediated radical chain reactions by benzeneselenol, generated in situ by reduction of diphenyl diselenide with tributyltin hydride, are described. The catalytic sequence is discussed in terms of polarity reversal catalysis of radical
Justin P Johnpeter et al.
Inorganic chemistry, 52(23), 13663-13673 (2013-11-20)
A series of cationic chalcogenolato-bridged diruthenium complexes [(η(6)-p-MeC6H4Pr(i))2Ru2(μ-EC6H5)3](+) (E = S, 1; E = Se, 2; E = Te, 3) has been obtained in ethanol from the reaction of (η(6)-p-MeC6H4Pr(i))2Ru2(μ-Cl)2Cl2 with benzenethiol, benzeneselenol, and sodium tellurophenolate, respectively. The thiolato and
Brittany Trang et al.
Journal of the American Chemical Society, 140(42), 13892-13903 (2018-09-29)
Silver metal exposed to the atmosphere corrodes and becomes tarnished as a result of oxidation and precipitation of the metal as an insoluble salt. Tarnish has so poor a reputation that the word itself connotes corruption and disrespectability; however, tarnishing
Nikolay V Orlov et al.
The Journal of organic chemistry, 79(24), 12111-12121 (2014-10-08)
A unique Ni-catalyzed transformation is reported for the one-pot highly selective synthesis of previously unknown monoseleno-substituted 1,3-dienes starting from easily available terminal alkynes and benzeneselenol. The combination of a readily available catalyst precursor, Ni(acac)2, and an appropriately tuned phosphine ligand
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.