추천 제품
분석
97%
양식
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
density
1.14 g/mL at 25 °C (lit.)
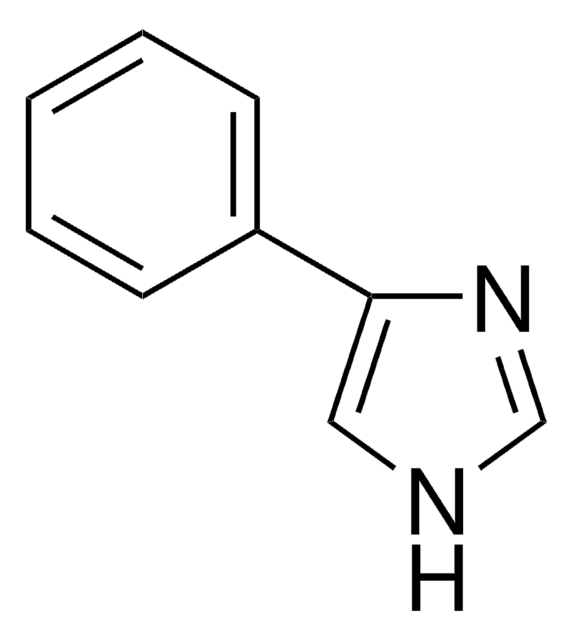
SMILES string
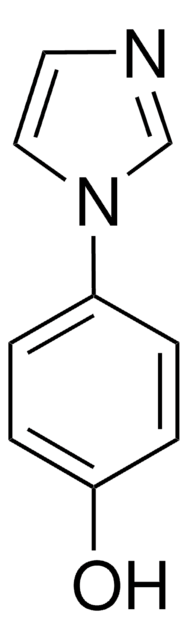
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
InChI key
SEULWJSKCVACTH-UHFFFAOYSA-N
일반 설명
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
애플리케이션
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Priyadarshini Balaraman et al.
Biochimica et biophysica acta. General subjects, 1863(2), 304-312 (2018-11-06)
The camphor-degrading microorganism, Pseudomonas putida strain ATCC 17453, is an aerobic, gram-negative soil bacterium that uses camphor as its sole carbon and energy source. The genes responsible for the catabolic degradation of camphor are encoded on the extra-chromosomal CAM plasmid.
Active-site structure analysis of recombinant human inducible nitric oxide synthase using imidazole.
R M Chabin et al.
Biochemistry, 35(29), 9567-9575 (1996-07-23)
Nitric oxide synthase catalyzes the pyridine nucleotide-dependent oxidation of L-arginine to nitric oxide and L-citrulline. It is a specialized cytochrome P450 monooxygenase that is sensitive to inhibition by imidazole. Steady-state kinetic studies on recombinant human inducible nitric oxide synthase (rH-iNOS)
D M Grant et al.
Biochemical pharmacology, 36(8), 1251-1260 (1987-04-15)
The nature of the cytochrome P-450-dependent enzyme reactions giving rise to four primary metabolites of caffeine was investigated using microsomes isolated from livers of human kidney donors. Metabolite formation proceeded at a lower rate than that predicted from in vivo
P R Kerklaan et al.
Journal of cancer research and clinical oncology, 111(3), 196-202 (1986-01-01)
The effect of the mixed-function oxidase inhibitor phenylimidazole (PI) and the amine oxidase inhibitors iproniazid (IPRO) and aminoacetonitrile (AAN) on the mutagenic activity of various carcinogens was determined in intrasanguineous host-mediated assays, using mice as hosts and E. coli 343/113
P Ammann et al.
Toxicology and applied pharmacology, 149(2), 217-225 (1998-05-08)
Chloroform is carcinogenic in rodents but is not mutagenic or DNA reactive. Chloroform-induced hepatocarcinogenesis in rodents is believed to be secondary to events associated with cytotoxicity and cell proliferation. Understanding the mechanisms of chloroform toxicity may provide insights into the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.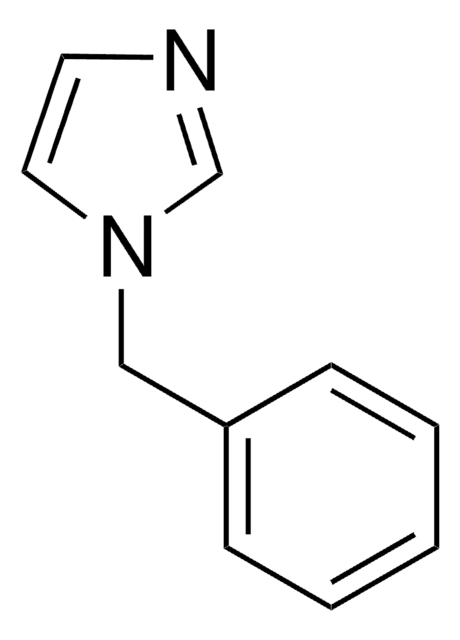



![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)