모든 사진(1)
About This Item
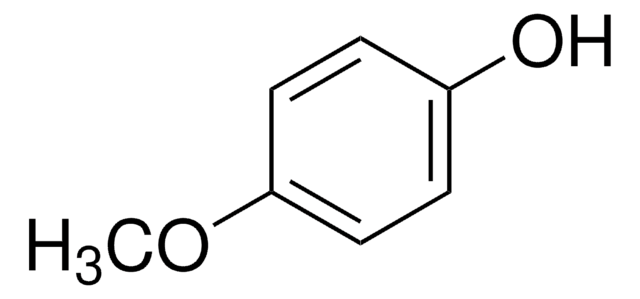
Linear Formula:
CH3OC6H4OH
CAS Number:
Molecular Weight:
124.14
Beilstein:
1209898
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
96%
양식
liquid
refractive index
n20/D 1.552 (lit.)
bp
113-115 °C/5 mmHg (lit.)
density
1.131 g/mL at 25 °C (lit.)
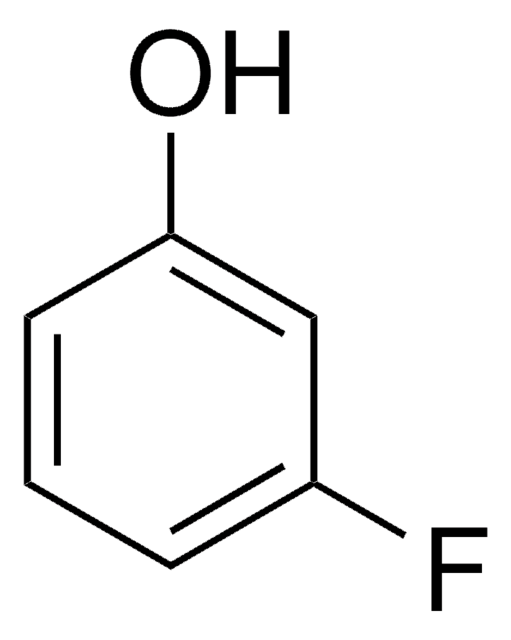
SMILES string
COc1cccc(O)c1
InChI
1S/C7H8O2/c1-9-7-4-2-3-6(8)5-7/h2-5,8H,1H3
InChI key
ASHGTJPOSUFTGB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Methoxyphenol was used in synthesis of:
- C(4) symmetric calix[4]resorcinarene
- 2-nitroso-5-methoxyphenol
- 6-methoxy-2(3H)-benzoxazolone
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
233.6 °F - closed cup
Flash Point (°C)
112 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Majid Y Moridani et al.
Chemico-biological interactions, 142(3), 317-333 (2002-11-28)
A tyrosinase-directed therapeutic approach for malignant melanoma therapy uses the depigmenting phenolic agents such as 4-hydroxyanisole (4-HA) to form cytotoxic o-quinones. However, renal and hepatic toxicity was reported as side effects in a recent 4-HA clinical trial. In search of
L G Fenoll et al.
Biophysical chemistry, 84(1), 65-76 (2000-03-21)
Tyrosinase hydroxylates 3-hydroxyanisole in the 4-position. The reaction product accumulates in the reaction medium with a lag time (tau) which diminishes with increasing concentrations of enzyme and lengthens with increasing concentrations of substrate, thus fulfilling all the predictions of the
Alejandro Cordero Vargas et al.
Organic letters, 5(20), 3717-3719 (2003-09-26)
[reaction: see text] A short synthesis of (+/-)-10-norparvulenone and (+/-)-O-methylasparvenone was developed starting from commercially available m-methoxyphenol, hinging on a xanthate-mediated addition-cyclization sequence for the construction of the alpha-tetralone subunit.
Mario C Foti et al.
The Journal of organic chemistry, 73(6), 2408-2411 (2008-02-26)
The m-methoxy group is normally electron-withdrawing (EW), sigma(m) = +0.12, sigma(m+) = +0.05. The strong EW activity of a phenoxyl radical's O* atom causes the m-methoxy group to become electron-donating (ED), sigma(m)(+) = -0.14. In valence bond terms, this can
McIldowie et al.
Organic letters, 2(24), 3869-3871 (2000-12-02)
The Lewis acid catalyzed condensation of 3-methoxyphenol with octanal produced the C(4) symmetric calix[4]resorcinarene 2, in high yield. Of the numerous stereo- and regioisomers possible, the rccc isomer with C(4) symmetry was the only product isolated (as a racemate). The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.