추천 제품
형태
liquid
Quality Level
농도
2.0 M in cyclohexane
bp
80 °C
density
0.775 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
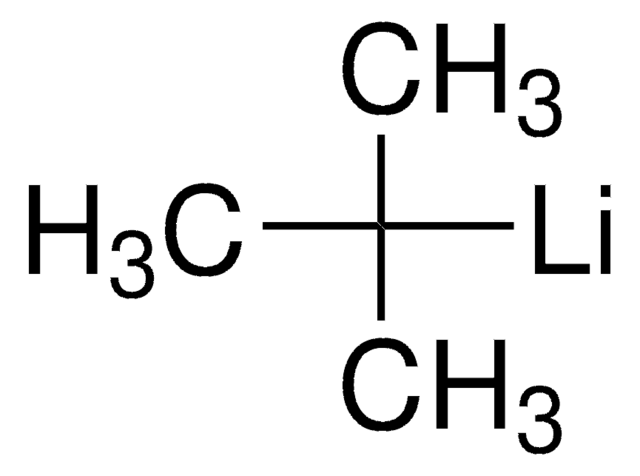
[Li]CCCC
InChI
1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
InChI key
MZRVEZGGRBJDDB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
n-Butyllithium (n-BuLi) is an organolithium reagent widely used as a strong base (superbase) in organic synthesis for the preparation of various chemical intermediates. It is also used as a reagent for lithium-halogen exchange and lithium-metal transmetalation reactions. n-BuLi is capable of lithiating carbon acids.
애플리케이션
n-Butyllithium (2.0 M in cyclohexane) can be used as:
- A polymerization initiator to synthesize polystyrenes by anionic polymerization of styrene.
- A strong base in the diastereoselective alkylation reactions.
- A reagent to synthesize 2-benzoylpyrroles by reacting benzaldehydes with di(1H-pyrrol-1-yl)zirconium(IV) chloride complex.
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
-0.4 °F - closed cup
Flash Point (°C)
-18 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Comparison of the synthetic utility of n-butyllithium and lithium diisopropylamide in the metalations of N, N-dialkyltoluamides.
Ludt RE, et al
The Journal of Organic Chemistry, 38(9), 1668-1674 (1973)
Arene-metal complexes. 13. Reaction of substituted (benzene) tricarbonylchromium complexes with n-butyllithium.
Card RJ and Trahanovsky WS.
The Journal of Organic Chemistry, 45(13), 2560-2566 (1980)
High-field proton NMR study of the aggregation and complexation of n-butyllithium in tetrahydrofuran.
McGarrity JF and Ogle CA.
Journal of the American Chemical Society, 107(7), 1805-1810 (1985)
Lithium intercalation via n-butyllithium of the layered transition metal dichalcogenides.
Dines MB.
Materials Research Bulletin, 10(4), 287-291 (1975)
Yuqiang Ma et al.
ACS nano, 9(7), 7383-7391 (2015-07-01)
Two-dimensional (2D) semiconducting monolayer transition metal dichalcogenides (TMDCs) have stimulated lots of interest because they are direct bandgap materials that have reasonably good mobility values. However, contact between most metals and semiconducting TMDCs like 2H phase WSe2 are highly resistive
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.