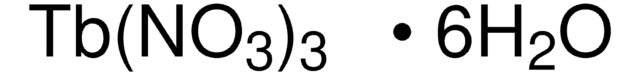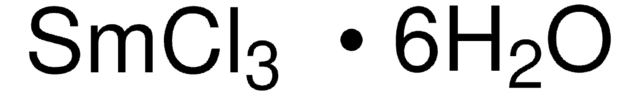298123
Samarium(III) nitrate hexahydrate
99.9% trace metals basis
동의어(들):
Samarium trinitrate, Trinitric acid samarium(III)·6hydrate
About This Item
추천 제품
Grade
for analytical purposes
Quality Level
분석
99.9% trace metals basis
양식
powder and chunks
반응 적합성
reagent type: catalyst
core: samarium
불순물
≤1500.0 ppm Trace Rare Earth Analysis
SMILES string
[Sm+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/3NO3.6H2O.Sm/c3*2-1(3)4;;;;;;;/h;;;6*1H2;/q3*-1;;;;;;;+3
InChI key
HDCOFJGRHQAIPE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- synthesis of nitrate precursor solution which can be used as a nanocatalyst in the solid oxide regenerative fuel cells.
- for the formation of coumarin derivative by von Pechmann condensation of phenol with ethyl acetoacetate in the presence of samarium complex as catalyst.
- for the preparation of samarium doped ceria, which can be used in the fabrication of electrolyte for fuel cells.
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
문서
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 298123-100G | 4061826657010 |
| 298123-25G | 4061826657027 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.