추천 제품
Quality Level
반응 적합성
reaction type: Grignard Reaction
농도
2.0 M in THF
density
0.962 g/mL at 25 °C
SMILES string
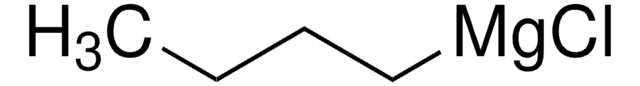
CCCC[Mg]Cl
InChI
1S/C4H9.ClH.Mg/c1-3-4-2;;/h1,3-4H2,2H3;1H;/q;;+1/p-1
InChI key
UUNGXYVLXYGZKH-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Butylmagnesium chloride (BuMgCl) is a Grignard reagent that can be used:
- To prevent the formation of passivating surface films on Mg electrodes.
- For the synthesis of lithium tributylmagnesate complex (n-Bu3MgLi) by reacting with n-BuLi.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
On the electrochemical behavior of magnesium electrodes in polar aprotic electrolyte solutions.
Lu Z, et al.
Journal of Electroanalytical Chemistry, 466(2), 203-217 (1999)
Tributylmagnesium ate complex-mediated novel bromine-magnesium exchange reaction for selective monosubstitution of dibromoarenes.
Iida T, et al.
Tetrahedron Letters, 42(29), 4841-4844 (2001)
B Pons et al.
Journal of chromatography. B, Biomedical sciences and applications, 716(1-2), 139-145 (1998-11-21)
One analytical procedure for the determination of ionic alkyllead in human urine has been studied. The system consists of the extraction of Me3Pb+, Et3Pb+ and Pb2+ at pH 9.0 with diethyldithiocarbamate to an organic phase. Then, the ionic compounds are
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.