추천 제품
분석
97%
양식
solid
bp
124-126 °C/20 mmHg (lit.)
mp
39-42 °C (lit.)
solubility
alcohol: soluble(lit.)
diethyl ether: soluble(lit.)
water: very slightly soluble(lit.)
작용기
bromo
carboxylic acid
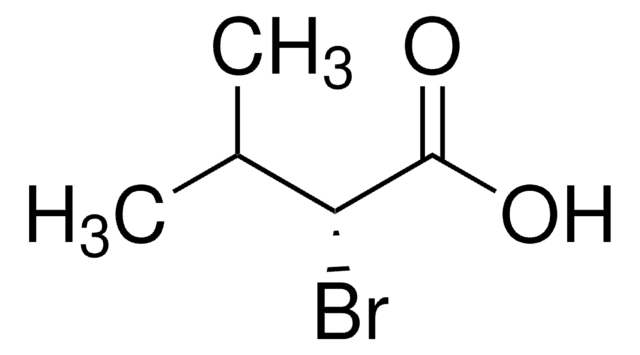
SMILES string
CC(C)C(Br)C(O)=O
InChI
1S/C5H9BrO2/c1-3(2)4(6)5(7)8/h3-4H,1-2H3,(H,7,8)
InChI key
UEBARDWJXBGYEJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Mechanism of action of methylamine on optically active 2-bromo-3-methylbutyric acid has been investigated.
애플리케이션
2-Bromo-3-methylbutyric acid has been used in the preparation of optically active N-methylvalines.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
224.6 °F - closed cup
Flash Point (°C)
107 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Depsipeptides.
Ovchinnikov YA, et al.
Russian Chemical Bulletin, 11(11), 1955-1961 (1962)
M Polhuijs et al.
Biochemical pharmacology, 44(7), 1249-1253 (1992-10-06)
Glutathione (GSH) conjugation of the separate enantiomers of five 2-bromocarboxylic acids and some of their urea derivatives by rat liver GSH transferases (GSTs) was studied. The liver cytosolic fraction conjugated all compounds, except for (R)-2-bromoisovaleric acid, with a variable degree
M Polhuijs et al.
Biochemical pharmacology, 38(22), 3957-3962 (1989-11-15)
The glutathione (GSH) conjugation of (R)-and (S)-alpha-bromoisovaleric acid (BI) in the rat in vivo, and its stereoselectivity, have been characterized. After administration of racemic [1-14C]BI two radioactive metabolites were found in bile: only one of the possible diastereomeric BI-GSH conjugates
J M Te Koppele et al.
The Biochemical journal, 252(1), 137-142 (1988-05-15)
The stereoselectivity of purified rat GSH transferases towards alpha-bromoisovaleric acid (BI) and its amide derivative alpha-bromoisovalerylurea (BIU) was investigated. GSH transferase 2-2 was the only enzyme to catalyse the conjugation of BI and was selective for the (S)-enantiomer. The conjugation
Chromatograms
application for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.